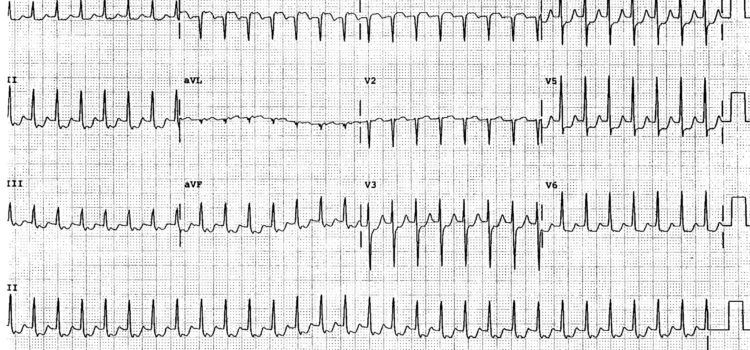
Most urgent care centers across the U.S. have taken a beating since social distancing recommendations and lockdown orders took hold. Patient visits fell sharply as patients who would typically come in for relatively minor complaints decided leaving home wasn’t worth the perceived risk. Finally, as parts of the country start to ease restrictions, data tracked by Experity show the industry is bouncing back strongly. Perhaps the most encouraging statistic is the difference in 7-day average …
Read More