Click Here to download the article PDF
Urgent message: Urinary tract infection is a common diagnosis in pediatric urgent care. As such, efforts must be made to ensure antibiotics are prescribed only when necessary and to decrease the rate of unnecessary prescribing.
Benjamin Klick, MD; Tammy Speerhas, DNP, FNP-C; Jessica Parrott, DNP, CPNP-PC, CNE; Jeffrey Bobrowitz, MD; Anne McEvoy, MD; Debra Conrad, MD, Jade Eves, PA-C; Theresa Guins, MD
Citation: Klick B, Speerhas T, Parrott J, Bobrowitz J, McEvoy A, Conrad D, Eves J, Guins T. A quality improvement project to improve management of urinary tract infections in a system of pediatric urgent care centers. J Urgent Care Med. 2023;17(9):23-29.
ARTICLE SUMMARY
A successful large scale QI project to decrease the prescription of unneeded antibiotics for UTIs was performed at a system of pediatric urgent care centers.
ABSTRACT
Background and objective: Urinary tract infections (UTIs) are a common problem in pediatric urgent care medicine. There are multiple quality improvement (QI) projects related to the management of UTIs documented in the pediatric literature. We developed a project to decrease the prescribing of ultimately unneeded antibiotics for possible UTIs in a pediatric urgent care setting. A similar project has not been described in the pediatric literature.
Methods: We first reviewed the charts of patients presenting to a system of pediatric urgent care centers with a possible UTI over a 2-year period. We then launched a QI project with three plan, do, study, act cycles to decrease the prescribing of antibiotics for patients who ultimately were found to not have a UTI based on urine culture results. We tracked the number of patients who needed to be started on antibiotics after their urgent care visit a balancing measure, and also tracked multiple secondary measures throughout the project. Balancing measures are tracked to make sure that unintended negative consequences do not occur from a QI project. In this case, the concern was that patients who should have been started on antibiotics for a UTI may have had a delay in care because of the project.
Results: The absolute percentage of antibiotics prescribed that were ultimately unneeded decreased by an absolute 15% during the project, and met special cause variation criteria. There was no special cause variation noted for our balancing measure. All of our secondary measures showed improvement during the project.
Conclusions: A large-scale QI project at a system of pediatric urgent care centers was able to decrease the unneeded prescription of antibiotics for possible UTIs.
INTRODUCTION
Urinary tract infections (UTIs) are a common problem in pediatric urgent care medicine. About 1.5% of children under 2 years old1 and over 6% of females under 6 years old2 have had at least one UTI. Current treatment recommendations for the diagnosis and management of patients below the age of 2 are based on 2011 American Academy of Pediatrics practice guidelines.3 There is less standardization of recommendations for pediatric patients over 2 years old, although European guidelines do exist which apply to children of all ages.4
There have been multiple previous quality improvement (QI) projects related to the diagnosis and management of pediatric UTIs. One project standardized proper ordering and collection of urine specimens as well as adherence to recommended antibiotic prescription for UTIs in an emergency department setting.5 Another developed a process map to decrease the incidence of missed UTI diagnoses6 in an emergency department setting. A third project sought to increase the rate of prescribing of narrow-spectrum antibiotics for UTIs in both an emergency department and urgent care setting.7 The authors are unaware of any reports in the literature of a pediatric urgent care quality improvement project focusing on decreasing the rate antibiotics were prescribed in cases where it was presumed that a patient had a UTI based on urinalysis, but where urine culture results were not consistent with a UTI.
This QI project took place at a system of pediatric urgent care centers associated with a large children’s hospital system in a catchment area of approximately 2 million patients. There were between three and four urgent care centers in the system during the study period, with a combined patient volume of over 90,000 visits per year at their busiest point. This urgent care system has a robust system of clinical care guidelines, including one for the diagnosis of UTIs. However, prior to initiation of the project, there had never been a quantitative evaluation of either the effectiveness of or adherence to this guideline.
METHODS
Project initiation
Planning for this quality improvement project began in late December 2019. Collecting data on the current diagnosis and management of UTIs at our urgent care centers was a major obstacle to project initiation. However, at the beginning of the COVID-19 pandemic, patient volumes declined precipitously in the pediatric population. This was used as an opportunity to launch the project.
The first step in our project was to review enough previous charts to decide if there were any issues in our diagnosis or management of UTIs that needed to be addressed. We chose to look at the 2 years prior to project onset to assure we would have sufficient data. As soon as all the data was collected it was analyzed. Once analysis was completed we began our plan, do, study, act (PDSA) cycles.
Initial data collection
There were a total of 6,548 patients who had a urine culture ordered between April 2018 and April 2020. All these charts were reviewed by an urgent care provider (physician, nurse practitioner, or physician assistant) and data were collected on all charts where both a urinalysis and urine culture were performed. Providers preferentially reviewed charts of patients who they had treated. The records of patients who had been treated by a provider who was no longer employed by our system of urgent care centers were divided up among the current providers. All data associated with the project were securely stored on RedCAP. Collected data included demographics, history, physical exam, laboratory findings and treatment.
Demographic information included age, sex, and insurance type. Insurance type was collected as a marker for socioeconomic status. The history and review of systems sections from each patient chart were used to answer if the patient had any vomiting, fever, or history of constipation. This historical information may affect the probability of a UTI. The physical exam section was reviewed to see if a urogenital physical exam was documented and if circumcision status was documented when appropriate. Laboratory findings included urinalysis, urine culture results as well as gonorrhea, chlamydia, and pregnancy testing when appropriate. The review included whether a patient was treated for a UTI, and if so, which antibiotic was chosen and duration of therapy. Lastly, we evaluated if proper hygiene practices were documented for all patients.
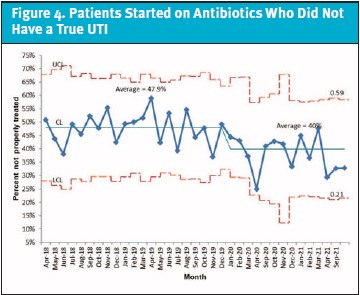
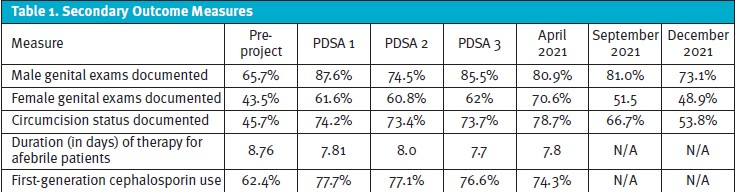
Using this initial data, several areas for improvement were identified. These included documentation of genital exams and circumcision status, use of a first-generation cephalosporin for treatment, and duration of therapy for afebrile patients. However, the largest area for improvement was in presumptive antibiotic treatment based on the initial urinalysis results: 47.9% of the patients treated for a presumed UTI proved to not meet criteria for continued treatment based on urine culture results. This became the primary target for this QI project, while the other measures mentioned above were also monitored throughout the project.
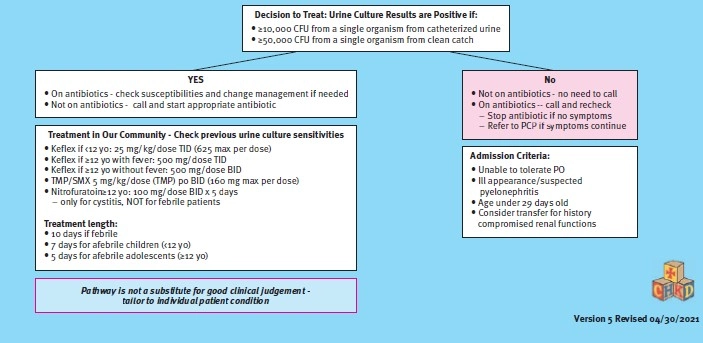
Our criteria for a UTI based on urine culture were at least 10,000 colony forming units of either a single or predominant organism for catheterized specimens or 50,000 colony forming units for a clean catch specimen. These cutoffs had been selected to minimize the risk of undertreatment of possible UTIs. Prior to launching our project, we submitted it for Eastern Virginia Medical School institutional review board approval, and it was determined that the project fell under QI and not research.
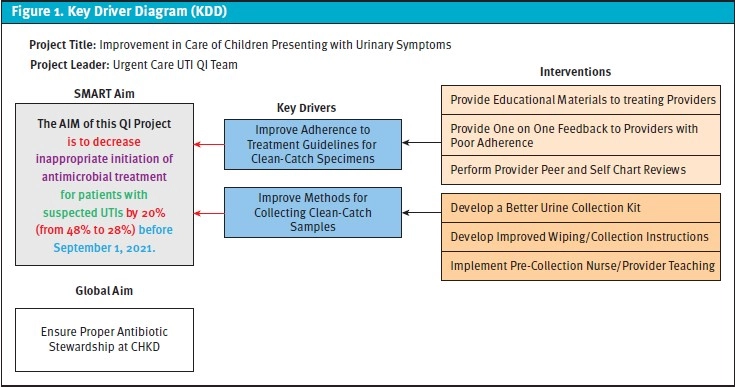
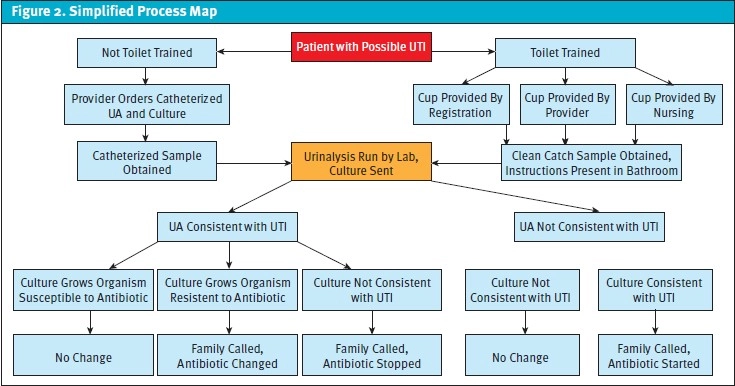
Our overall project aim statement was to decrease the number of patients who were started on antibiotics for a presumed UTI at the time of their urgent care visit and then had a subsequent negative culture result by an absolute 20%, from 48% to 28% within a year. Our system of urgent care centers has always called patients in cases when antibiotics needed to be stopped based on urine culture results, and this practice was not affected by this QI project. Our main balance measure would be patients who needed to be started on antibiotics based on urine culture results. We developed a key driver diagram (Figure 1) and process map (Figure 2) to aid us in this goal. Major areas for potential improvement that were identified included provider adherence to treatment guidelines for presumed UTIs and clean-catch urinalysis collection technique. Updating our current guidelines regarding treatment of a presumed UTI was another area of focus. We then began the first of three plan, do, study, act cycles.
PLAN, DO, STUDY, ACT CYCLE 1
PDSA cycle 1 started in September 2020 and finished in October 2020. It focused on decreasing contamination of clean-catch urine samples with educational interventions. Proper urine collection technique was reviewed at pre-shift huddles with nursing staff and providers, as well as with staff educational poster boards placed at each center. A new urine collection kit with extra wipes was developed and placed in all patient rooms. Large posters with collection instructions were placed in bathrooms. Starting with charts from October 2020, providers began a real-time chart review of their peers and stopped reviewing their own charts with the goal of learning from how others practiced. An additional aspect to the PDSA 1 cycle was a reflective practice survey that was distributed to all participating providers. The survey included questions regarding provider demographics, a reflective practice questionnaire, and perceptions on clinical practice.
Utilizing this interprofessional (physicians, nursing practitioners and physician assistants) reflective practice methodology, several significant and positive findings were discovered regarding perceived provider improvement of care and demonstrated value in the interprofessional reflective process.
Exploration of these results were presented as a poster at the 2022 Society for Pediatric Urgent Care conference. Additionally, we presented an overview of the project in September 2020 at our health system-wide quality and safety meeting, just as the first PDSA cycle was starting.
PLAN, DO, STUDY, ACT CYCLE 2
PDSA cycle 2 started in November 2020 and ran through December 2020. It continued and expanded the interventions from PDSA 1. Urine collection instructions were translated into Spanish and laminated handouts with instructions were created for patient rooms. Finally, an email was sent to all providers reminding them to follow divisional guidelines regarding the treatment of presumed UTIs.
PDSA 3
PDSA 3 started in January 2021 and ran through March 2021. Prior to starting the third cycle, our divisional guidelines regarding criteria for a presumed UTI were updated based on the information gathered from the chart review. Our health system’s antibiogram shows excellent sensitivity to first-generation cephalosporins by common UTI pathogens, which is why it has remained our recommended first-line treatment option on our guideline. The updated guideline (Figure 3) recommended empiric treatment for a UTI only if both positive leukocyte esterase (at least moderate on dipstick) and white blood cells (at least 10-25 per high-powered field) were present, rather than if either of these criteria were met.
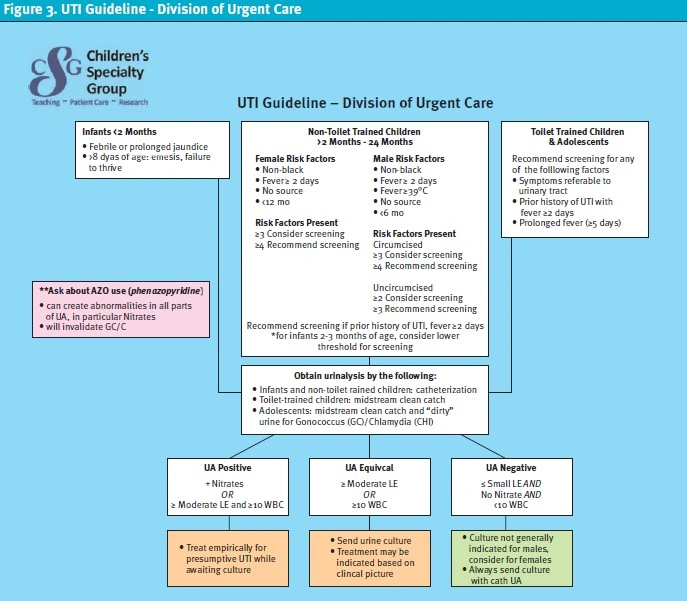
Empiric treatment for patients with positive nitrite on dipstick was still recommended, but a note about possible false positive nitrites from use of phenazopyridine was included. Recommendations for when to obtain a urine culture if the urinalysis was negative were added, as well. These recommendations were to always obtain a culture for catheterized specimens and to consider a urine culture for female patients. For males with a normal urinalysis, it was recommended that a urine culture is generally not indicated. These divisional guideline changes and associated provider education were the major interventions in this cycle.
LATER STEPS
In April 2021, the formal PDSA cycles stopped due to a significant increase in patient volumes related to COVID-19 trends in the pediatric population. Project monitoring continued periodically through December 2021. Fewer data were collected during this time; in particular, mean antibiotic duration for afebrile patients and use of first-generation cephalosporins were no longer tracked. This is because improvement in these measures had occurred in the first PDSA cycle and had remained consistent throughout the other PDSA cycles. During that time, project updates were presented twice at the health system-wide quality and safety meetings. Our findings were also presented at our institution’s annual research day and as a podium presentation at the 2021 Society for Pediatric Urgent Care conference.
Results
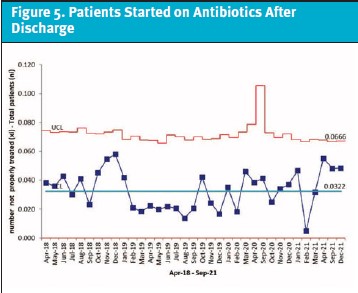
The initial aim of obtaining an absolute 20% reduction of unneeded antibiotic initiation within a year was not achieved. However, this number did drop to 32.8%, an absolute reduction of approximately 15%. When plotted on a p-chart, 15 consecutive data points starting when the project was first discussed prior to the COVID-19 pandemic were also below the original 48% average, which meets special cause variation (Figure 4). The center line of our p-chart was adjusted at the end of PDSA 3 to reflect improvement since the time that the project was first discussed. The percentage of patients who needed to be started on antibiotics after their urgent care visit, again plotted on a p-chart, did not meet special cause variation, which was also the desired outcome (Figure 5). All our secondary outcomes also improved (Table 1). Periodic data collection and analysis will continue to take place, and data from summer 2022 is currently being analyzed.
DISCUSSION
The COVID-19 pandemic created many problems in urgent care centers and in our own health system, but it also created opportunities. The initial substantial decrease in patient volumes at the onset of the pandemic allowed the launch of initiatives that would not normally be feasible. It is also an example of how a system-wide project requiring effort from all clinicians can succeed. Every outcome measure that was tracked during this project improved without any significant worsening of our balancing measure. We hope to see this positive change sustained and intend to use the lessons learned as a foundation for other division wide quality improvement projects.
Manuscript submitted November 17, 2022; accepted March 24, 2023.
REFERENCES
- Jakobsson B, Esbjörner E, Hansson S. Minimum incidence and diagnostic rate of first urinary tract infection. Pediatrics. 1999;104(2 Pt 1):222-226.
- Mårild S, Jodal U. Incidence rate of first-time symptomatic urinary tract infection in children under 6 years of age. Acta Paediatr. 1998;87(5):549-552.
- Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610.
- Stein R, Dogan HS, Hoebeke P, et al. Urinary tract infections in children: EAU/ESPU guidelines. Eur Urol. 2015;67(3):546-558.
- Walters EM, D’Auria J, Jackson C, et al. An ambulatory antimicrobial stewardship initiative to improve diagnosis and treatment of urinary tract infections in children. JtComm J Qual Patient Saf. 2019;45(12):829-837.
- Black M, Singh V, Belostotsky V, et al. Process mapping in a pediatric emergency department to minimize missed urinary tract infections. Int J Pediatr. 2016;2016:2625870.
- Poole NM, Kronman MP, Rutman L, et al. Improving antibiotic prescribing for children with urinary tract infection in emergency and urgent care settings. Pediatr Emerg Care. 2020;36(6): e332-e339.
ACKNOWLEDGMENTS
The authors state that this was an unfunded project. Benjamin Klick participated as a co-project leader throughout the duration of the project. He was on the project leadership team during project planning and implementation. He helped with data collection and analysis. He was the primary author of the manuscript. Tammy Speerhas participated as a co-project leader throughout the duration of the project. She was on the project leadership team during project planning and implementation. She helped with data collection and analysis, and helped with manuscript editing and revision. Jessica Parrott, Jeffrey Bobrowitz and Anne McEvoy were on the project leadership team during project planning and implementation. They helped with data collection and analysis, and helped with major manuscript revisions. Debra Conrad and Jade Eves were on the project leadership team during project planning and implementation. They helped with data collection and analysis, and reviewed the manuscript. Theresa Guins participated as a co-project leader throughout the duration of the project. She was on the project leadership team during project planning and implementation. She reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Author affiliations: Benjamin Klick, MD, Children’s Hospital of the King’s Daughters, Norfolk, VA; Eastern Virginia Medical School, Norfolk, VA. Tammy Speerhas, DNP, FNP-C, Children’s Hospital of the King’s Daughters, Norfolk, VA; Eastern Virginia Medical School, Norfolk, VA; and Old Dominion University, Norfolk, VA. Jessica Parrott, DNP, CPNP-PC, CNE, Children’s Hospital of the King’s Daughters, Norfolk, VA; Eastern Virginia Medical School, Norfolk, VA; and Old Dominion University, Norfolk, VA. Jeffrey Bobrowitz, MD, Children’s Hospital of the King’s Daughters, Norfolk, VA; Eastern Virginia Medical School, Norfolk, VA. Anne McEvoy, MD, Children’s Hospital of the King’s Daughters, Norfolk, VA; Eastern Virginia Medical School, Norfolk, VA. Debra Conrad, MD, Children’s Hospital of the King’s Daughters, Norfolk, VA. Jade Eves, PA-C, Children’s Hospital of the King’s Daughters, Norfolk, VA; Eastern Virginia Medical School, Norfolk, VA. Theresa Guins, MD, Children’s Hospital of the King’s Daughters, Norfolk, VA; Eastern Virginia Medical School, Norfolk, VA. The authors have no relevant financial relationships with any commercial interest.

