Urgent message: Low-dose naltrexone (LDN) is becoming more common as a treatment option for pain and thus will be increasingly prevalent in patients presenting to the urgent care setting. A thorough medication history, prioritization of non-opioid treatment options, and timely referral or transfer for severe uncontrolled pain are important considerations in the management of patients using low-dose naltrexone.
Ting-Hsuan Chiang, MD; Kenneth Schmitt, BS; Ariana Nelson, MD
INTRODUCTION
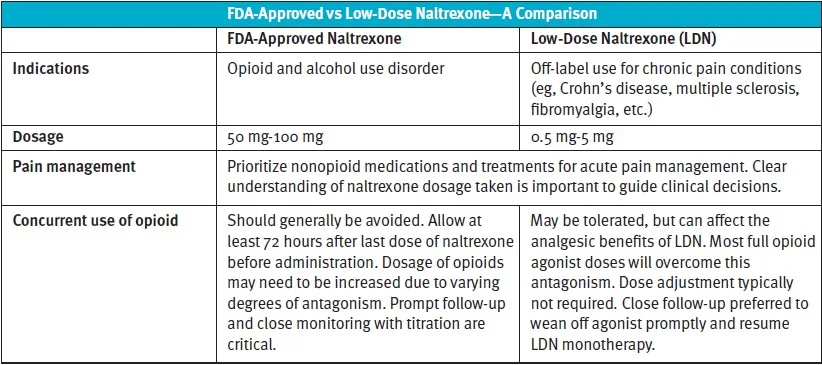
Naltrexone is an opioid receptor antagonist approved by the Food and Drug Administration for the treatment of alcohol use disorder and opioid use disorder at high doses of 50 mg to 100 mg, daily. By binding to opioid receptors, naltrexone blocks the effects and reduces cravings for opioid and alcohol consumption.1,2
In contrast, low-dose naltrexone (LDN), with doses ranging from 1 mg to 5 mg per day, has gained popularity in recent years due to its demonstrated efficacy in the management of chronic pain conditions. This novel pharmacologic therapy not only offers a safer alternative to opioid-based medications, but also has fewer side effects.
With the increased use of LDN as an off-label treatment for several chronic inflammatory diseases, urgent care providers may encounter patients on LDN seeking treatment for pain. However, due to its potential effect on the opioid response, acute pain management in these patients is an area not yet explored in the literature.
This review article focuses on current evidence of LDN for chronic pain and highlights pain management for this specific patient group in the urgent care setting.
PAIN MANAGEMENT IN URGENT CARE
Urgent care centers have rapidly expanded in the past two decades,3 with patient visits increasing each year. Pain is one of the most common chief complaints in urgent care clinics,4 and prescription of opioids in urgent care is not uncommon. A retrospective study examining urgent care in-clinic opioid prescriptions found that fractures, joint dislocations, musculoskeletal pain, and abdominal pain are the most common diagnoses that led to opioid prescription.5
Generally, the concomitant use of opioid and LDN should be avoided.6 LDN is unlikely to precipitate withdrawal symptoms for patients on opioids at these low doses, but it is prudent to recommend that patients on continuous opioid therapy wean entirely off opioids before initiating LDN. Even at low doses, there is a theoretical risk that the blockage of opioid receptors can reduce the effect of opioid agonists to varying degrees.1 However, the more likely scenario is that the disturbance of the endogenous opioid system by exogenous opioid agonist administration will interfere with the analgesic benefits of LDN and thus the two should not be given together. Chronic use of naltrexone is known to increase opioid sensitivity through upregulation of mu receptors in the CNS.7 Although current evidence on opioid hypersensitivity of naltrexone has not been studied in LDN, this potential upregulation increases the complexity of corresponding clinical decisions.
To avoid unnecessary use of opioids, urgent care management for patients on LDN should prioritize nonopioid medications and nonpharmacologic therapies. Nonpharmacologic therapies, including nerve blocks and local anesthetic infiltration, may not be feasible in the urgent care setting. Therefore, nonopioid medications such as NSAIDs should be initially considered. Prompt referral to pain management facilities or urgent transfer to an emergency department may be necessary in some cases if severe pain cannot be adequately addressed, in which case opioid agonists should be employed to ensure appropriate mitigation of patient suffering.
The initiation of opioids in acute pain depends upon the etiology and severity of pain. In situations where opioid-based analgesics are deemed necessary, consider using short-acting, high-affinity, full opioid agonists to overcome any potential opioid receptor blockade of LDN. For the FDA-approved dose of oral naltrexone, which is much higher than LDN, that is used to treat alcohol and opioid use disorder, it is considered safe to initiate opioids after discontinuing naltrexone for at least 72 hours.8 To overcome the antagonism, patients often require increased dose of opioids and slow titration to effect. As the effect of high-dose naltrexone wanes over time, the opioid agonist should also be decreased to avoid respiratory depression or sedation. This concomitant titration should be conducted with caution and close interdisciplinary coordination, given the potential of patient hypersensitivity to opioid effects with long-term use of naltrexone.
Theoretically, at the lower doses used for analgesic benefit (such as LDN <5 mg), usage of opioid agonists can be much more lenient. Initial doses of opioids for these patients, in contrast to those on full-dose naltrexone, typically do not need to be increased to overcome antagonism. While the co-administration of LDN and opioid agonists has been investigated,9,10 there are insufficient data on the dosage effect of LDN and concurrent use of opioids.
When initiating opioids, it is important to understand the dose of naltrexone patients are taking and be aware of varying opioid sensitivity over time to guide clinical decisions. Regardless of the dose of naltrexone patients are taking, conservative dosing and close monitoring with follow-up should be prioritized. In addition to the dose, other factors to take into consideration include time of the last dose and any concurrent opioid use.
Pain management in patients on LDN is further complicated by its frequent absence on electronic health record medication lists, as it is often acquired from compounding pharmacies. Additionally, some providers may prescribe higher doses of naltrexone and instruct patients to break pills into smaller portions in order to get coverage from insurance companies if the out-of-pocket cost is difficult for the patient to manage. Reconfirming dose and frequency of LDN administration with patients while acquiring their medication history is therefore critical.
Evidence on Chronic Pain Conditions
Randomized trials have demonstrated efficacy and shown promising safety profiles11 on the use of off-label LDN for several chronic pain conditions and autoimmune diseases. Current evidence mainly supports the efficacy of LDN for multiple sclerosis, Crohn’s disease, and fibromyalgia. Benefits of LDN on outcomes, such as improved quality of life, pain, overall stable disease state, and lessened fatigue and anxiety were identified in multiple retrospective and small prospective studies.12-14 Larger, longer duration randomized trials are warranted for definite conclusions on the efficacy of LDN for different chronic conditions.15
Multiple sclerosis
Multiple sclerosis (MS) is one of the earliest and most studied chronic diseases with regard to LDN. Clinical studies reported reduced relapse rate, slowed disease progression, stabilized quality of life, and reduced fatigue among MS patients started on LDN.13,14,16,17 It quickly gained popularity after a Norwegian documentary in 2013, with MS patients claiming significantly improved function after the use of LDN. According to the Norwegian prescription database (NorPD), after this documentary, the number of naltrexone users quickly grew from less than 20 to more than 15,000. With data from NorPD, a study found a significant reduction in opioid consumption and NSAID use among long-term LDN users.6 While not all clinical studies prove efficacy of LDN, they all have found that it is well-tolerated, with no documented serious adverse events and few side effects.18 Among the reported side effects of LDN, headache is the most common. Others include insomnia and nightmares.17
Crohn’s disease
It is hypothesized that regulation of the innate opioid system could be effective in treating Crohn’s disease due to the overexpression of mu opioid receptor by CD4+ and CD8+ T-lymphocytes. Therefore, the opioid rebound effect of LDN may contribute to the effects of LDN on Crohn’s disease.20
Although limited in sample size, several clinical trials have shown promising results of LDN for the reduction of Crohn’s disease activity index (CDAI).20,21 Studies reported more than 80% of patients responded with decreased CDAI after using daily LDN. In a pilot study looking at pediatric patients, remission was reported in 25% of patients and 67% had only mild disease activity after an 8-week course of LDN.22 Some documented side effects of LDN include fatigue, sleep disturbance, nausea, and headache.18 However, these side effects are infrequent and usually mild.12
Fibromyalgia
It is generally believed that the endorphin rebound effect from transient blockade of opioid receptors contributes to the attenuation of pain in fibromyalgia.23 Several studies and case reports have shown improvement of pain, physical function, and mood in fibromyalgia patients with the use of LDN.23-25 A crossover trial of 10 women found the use of LDN increased mechanical and heat pain thresholds in patients.24 They also reported that response to LDN correlated directly to ESR, suggesting that LDN may be useful in those with signs of inflammation. As fibromyalgia is a disorder of the CNS with a neuroimmune component, the immunomodulating benefit of LDN has been proposed to play a potential role in the pain attenuating effect.12 Another crossover trial of eight women found reduced plasma concentrations of pro-inflammatory cytokines and overall symptoms when treated with 8 weeks of LDN, further supporting the hypothesis of LDN as an anti-inflammatory medication for fibromyalgia.26 Similar to that of MS and Crohn’s disease, current data suggest excellent safety and tolerability of LDN for fibromyalgia.24,25
LOW-DOSE NALTREXONE FOR CHRONIC PAIN
Pharmacodynamics and Pharmacokinetics
As a competitive, reversible opioid receptor antagonist, naltrexone has a high affinity for μ-opioid greater than κ-opioid receptors.2,3Naltrexone is absorbed orally, and is then metabolized largely via first-pass metabolism in the liver by the enzyme non-cytochrome dehydrogenase to form its active metabolite, 6β-naltrexol.
When orally administered, naltrexone and 6β-naltrexol have a half-life of 4 and 13 hours, respectively. Following intramuscular administration, the half-life increases to 5 to 10 days for both unmetabolized naltrexone and its metabolite.
Naltrexone shares a similar pharmacologic profile with naloxone but diverges when comparing certain pharmacokinetic properties, including a notable increase in oral bioavailability and half-life of the former.24 Though its elimination occurs primarily via renal filtration and excretion, naltrexone dosage adjustments have been deemed unnecessary for patients with mild renal impairment.12 Still, further studies are necessary regarding severe renal impairment, and caution is recommended when treating the end-stage renal disease patient populations with naltrexone regimens.12
Mechanism of action
The mechanism and application of low-dose naltrexone (LDN) centers on its multimodal cellular effects that is dosage-dependent.18 Several pathways found in animal and in vitro studies are believed to contribute to the unique analgesic, anti-inflammatory, and immunomodulatory properties of LDN due to varying dose-dependent pharmacological targets.27
Naltrexone’s nonlinear analgesic relationship between doses and pharmacological outcomes can partially be understood by its effects on the μ-opioid receptor (MOR) G protein-coupled receptor (GPCR). As a semisynthetic opioid antagonist, naltrexone works similarly to many other prescribed opioids by targeting MORs largely found on neurons linked to pain signaling.18,28,29Further studies have suggested a relationship between chronic administration of opioids and shifts in MOR GPCR in partial favor of a Gs-coupled rather than Gi-coupled response.30
This understanding holds clinical significance with the display of hyperalgesia, tolerance, and dependence in the setting of chronic MOR stimulation. However, varying doses of certain opioids have shown differing preferences in GPCR response.31From this, the concept of lower-dosage opioid treatment in favor of Gi-couple partiality has been explored. Animal studies on mice have demonstrated that the application of low perfusion doses in combination with opioid treatment has led to notable reductions in action potential propagation and tolerance.31
A necessary element in the function and understanding of naltrexone’s downstream cellular effects includes the recognition of a scaffolding protein filament associated with MORs called filamin-A (FLNA).31 When bound by naltrexone, the MOR Gi-coupling is favored over the Gs-coupling response, promoting the analgesic effects of administered opioid agonists. However, FLNA also has a binding affinity for opioid antagonists and, with the saturation of both agonist and antagonist binding sites, the above-mentioned promising opioid agonist effects are reduced.
Opioid rebound effect
LDN has also been shown to induce the increased assembly of endogenous opioids in contrast to higher standard doses of naltrexone.32-34 Naltrexone administration at doses less than 0.5 mg/kg have been linked to increased levels of endogenous levels of endorphin and metenkephalin.12,15-17Additional literature suggests an associated increase in opioid receptor expression in relation to this “opioid rebound” effect.32,35,36,37
Anti-inflammatory effects of naltrexone
Naltrexone also shows promising anti-inflammatory effects at lower dose regimens. This is likely induced through interactions with toll-like receptor 4 (TLR4), a key receptor in proinflammatory downstream cellular signaling including the release of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α).38
Though opioid medications have been linked previously to the stimulation of proinflammatory effects via TLR4 signaling, low-dose naltrexone has paradoxically been correlated with the promotion of anti-inflammatory effects through the inhibition of TLR4 signaling.39,40 Given the high occurrence of TLR4 among microglial cells, LDN potentially possesses additional properties that are immunosuppressive and diminish neuropathic pain. Naltrexone and naloxone have both been shown to cross the blood-brain barrier and, therefore, can conceivably affect central and peripheral immune cell reactivity.40Prior in vivo animal studies have highlighted the plausibility of treating neuropathic pain with the inhibition of TLR4 receptors through the administration of naltrexone and naloxone.39,41,42 Further studies have confirmed LDN’s increased affinity for TLR4 receptors, including a minimal selectivity for dopamine, noradrenaline, and serotonin transporters.43,44 Such findings provide further support for naltrexone as an alternative treatment for neuropathic pain.
Opioid growth factor-opioid growth factor receptor axis regulation
LDN has additionally been reported to have an influence on the opioid growth factor-opioid growth factor receptor (OGF-OGFr) axis. This can be explained by LDN’s transient competitive inhibition of OGFr, resulting in a compensatory feedback response to increase OGF and OGFr expression.45 Low quantities of naltrexone lead to a short-lived inhibition of OGFr that is rapidly processed prior to subsequent doses, producing a period of amplified OGF and OGFr expression and interaction.25
Dosage and expense
The standard, FDA-approved dose of naltrexone for opioid use disorder and alcohol use disorder is between 50 mg and 100 mg. Therefore, the current commercially available naltrexone oral tablet is 50 mg.
Dosage for such disorders can be further reduced via a tablet cutter and started as low as 25 mg orally to allow for close follow-up and observation for adverse effects or withdrawal symptoms. Naltrexone is also available via intramuscular injection (380 mg), recommended for patients who would benefit from naltrexone treatment of SUD or AUD but find it difficult to be adherent to a daily oral administration regimen.46
Intramuscular naltrexone, in comparison to its oral form, is significantly longer acting.47 Therefore, clinicians will need to maintain vigilance in opioid titration for 5 to 10 days given this longer period of antagonist medication washout. In terms of LDN, dosages range between 0.5 mg to 5 mg, depending on individual patient requirements and responses.18
Such low-dose prescriptions have amassed support for off-label use in a myriad of chronic pain syndromes; however, commercially available LDN continues to be absent on formularies. Lower doses of naltrexone are readily available via compounding pharmacies and can be individualized to patients’ needs.
Additionally, while medication pricing fluctuates extensively across the nation, the average cost of LDN, including medication compounding, has previously been reported as $35 per month. Although patients must pay this fee out of pocket, this is much lower in comparison to several medications used to treat specific chronic pain diseases.1 In the current healthcare climate, amongst an opioid epidemic and ever-increasing medical expenses with many having inadequate pain control on higher opioid regimens, alternative strategies considering both optimal pain relief and healthcare expenditure are highly desired.
CONCLUSION
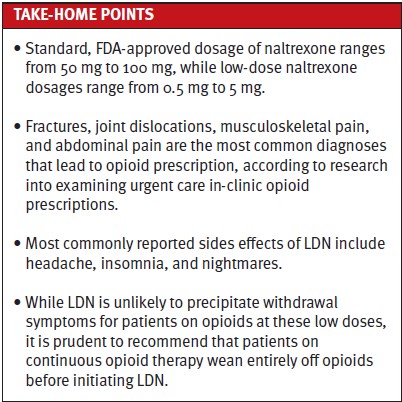
LDN has been defined as the regular administration of naltrexone, usually on a daily basis, in doses that range from 0.5 to 5 mg. LDN has shown promising results in a number of chronic pain conditions, including multiple sclerosis, Crohn’s disease, and fibromyalgia. For patients with acute pain who are taking LDN, nonopioid analgesics should be prioritized. When opioids are necessary, FDA-approved naltrexone doses ranging from 50 to 100 mg for the treatment of alcohol- and opioid-use disorder often involve a higher dose to overcome antagonism and cautious titration to take effect. In contrast, off-label LDN regimens typically do not require increased dosages of opioids and are largely dependent on patient-specific tolerance. Close monitoring and prompt follow-up are critical when concurrently administering opioids and naltrexone. As research continues on its application and benefits, LDN treatment among the urgent care population is expected to increase. As such, information on management of LDN regimens in the urgent care setting is needed in order to continue to support this patient population.
References
- Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33(4):451-459.
- Toljan K, Vrooman B. Low-dose naltrexone (LDN)-review of therapeutic utilization. Med Sci (Basel). 2018;6(4):82.
- Poon SJ, Schuur JD, Mehrotra A. Trends in visits to acute care venues for treatment of low-acuity conditions in the United States from 2008 to 2015. JAMA Internal Medicine. 2018;178(10):1342-1349.
- Rothstein R, Zhen K, Kim RY, Olympia RP. Acuity-appropriate triage of chief complaints found on urgent care center organization websites. Am J Emerg Med. 2021;43:276-280.
- Calcaterra SL, Lou Y, Everhart RM, et al. Association between in-clinic opioid administration and discharge opioid prescription in urgent care: a retrospective cohort study. J Gen Intern Med. 2021;36(1):43-50.
- Raknes G, Småbrekke L. Low-dose naltrexone and opioid consumption: a drug utilization cohort study based on data from the Norwegian prescription database. Pharmacoepidemiol and Drug Saf. 2017;26(6):685-693.
- Suzanne Zukin R, Sugarman JR, Fitz-Syage ML, Gardner EL, et al. Naltrexone-induced opiate receptor supersensitivity. Brain Res. 1982;245(2):285-292.
- Vickers AP, Jolly A. Naltrexone and problems in pain management. BMJ. 2006;332(7534):132-133.
- Davis M, Goforth HW, Gamier P. Oxycodone combined with opioid receptor antagonists: efficacy and safety. Expert Opin Drug Saf. 2013;12(3):389-402.
- Burns LH, Wang H-Y. Ultra-low-dose naloxone or naltrexone to improve opioid analgesia: the history, the mystery and a novel approach. Clinical Medicine Insights: Therapeutics. 2010;2:CMT.S4870.
- Ludwig MD, Turel AP, Zagon IS, McLaughlin PJ. Long-term treatment with low dose naltrexone maintains stable health in patients with multiple sclerosis. Mult Scler J Exp Transl Clin. 2016;2:2055217316672242.
- Patten DK, Schultz BG, Berlau DJ. The safety and efficacy of low-dose naltrexone in the management of chronic pain and inflammation in multiple sclerosis, fibromyalgia, Crohn’s disease, and other chronic pain disorders. Pharmacotherapy. 2018;38(3):382-389.
- Cree BAC, Kornyeyeva E, Goodin DS. Pilot trial of low-dose naltrexone and quality of life in multiple sclerosis. Ann Neurol. 2010;68(2):145-150.
- McLaughlin PJ, Odom LB, Arnett PA, et al. Low-dose naltrexone reduced anxiety in persons with multiple sclerosis during the COVID-19 pandemic. Int Immunopharmacol. 2022;113(Pt B):109438.
- Sharafaddinzadeh N, Moghtaderi A, Kashipazha D, et al. The effect of low-dose naltrexone on quality of life of patients with multiple sclerosis: a randomized placebo-controlled trial. Mult Scler. 2010;16(8):964-9.
- Raknes G, Småbrekke L. Low dose naltrexone in multiple sclerosis: effects on medication use. A quasi-experimental study. PLoS One. 2017;12(11):e0187423.
- Turel AP, Oh KH, Zagon IS, McLaughlin PJ. Low dose naltrexone for treatment of multiple sclerosis: a retrospective chart review of safety and tolerability. J Clin Psychopharmacol. 2015;35(5):609-611.
- Kim PS, Fishman MA. Low-dose naltrexone for chronic pain: update and systemic review. Curr Pain Headache Rep. 2020;24(10):64.
- Philippe D, Dubuquoy L, Groux H, et al. Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest. 2003;111(9):1329-1338.
- Smith JP, Stock H, Bingaman S, et al. Low-dose naltrexone therapy improves active Crohn’s disease. Am J Gastroenterol. 2007;102(4):820-828.
- Parker CE, Nguyen TM, Segal D, et al. Low dose naltrexone for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2018;4(4):Cd010410.
- Smith JP, Field D, Bingaman SI, et al. Safety and tolerability of low-dose naltrexone therapy in children with moderate to severe Crohn’s disease: a pilot study. J Clin Gastroenterol. 2013;47(4):339-345.
- Metyas S, Chen CL, Yeter K, et al. Low dose naltrexone in the treatment of fibromyalgia. Curr Rheumatol Rev. 2018;14(2):177-180.
- Younger J, Mackey S. Fibromyalgia symptoms are reduced by low-dose naltrexone: a pilot study. Pain Med. 2009;10(4):663-672.
- Younger J, Noor N, McCue R, Mackey S. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65(2):529-538.
- Parkitny L, Younger J. Reduced pro-inflammatory cytokines after eight weeks of low-dose naltrexone for fibromyalgia. Biomedicines. 2017;5(2):16.
- Calabrese EJ. Hormetic mechanisms. Crit Rev Toxicol. 2013;43(7):580-606.
- Loh HH, Liu HC, Cavalli A, et al. mu Opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res. 1998;54(2):321-326.
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267-284.
- Largent-Milnes TM, Guo W, Wang HY, et al. Oxycodone plus ultra-low-dose naltrexone attenuates neuropathic pain and associated mu-opioid receptor-Gs coupling. J Pain. Aug 2008;9(8):700-713.
- Shen KF, Crain SM. Dual opioid modulation of the action potential duration of mouse dorsal root ganglion neurons in culture. Brain Research. 1989;491(2):227-242.
- Rahn KA, McLaughlin PJ, Zagon IS. Prevention and diminished expression of experimental autoimmune encephalomyelitis by low dose naltrexone (LDN) or opioid growth factor (OGF) for an extended period: Therapeutic implications for multiple sclerosis. Brain Res. 2011;1381:243-253.
- Robinson A, Wermeling DP. Intranasal naloxone administration for treatment of opioid overdose. Am J Health Syst Pharm. 2014;71(24):2129-2135.
- Immonen JA, Zagon IS, McLaughlin PJ. Selective blockade of the OGF-OGFr pathway by naltrexone accelerates fibroblast proliferation and wound healing. Exp Biol Med (Maywood). 239(10):1300-1309.
- Tempel A, Gardner EL, Zukin RS. Neurochemical and functional correlates of naltrexone-induced opiate receptor up-regulation. J Pharmacol Exp Ther. 232(2):439-444.
- Zukin RS, Sugarman JR, Fitz-Syage ML, et al. Naltrexone-induced opiate receptor supersensitivity. Brain Res. 1982;245(2):285-292.
- Miskoff JA, Chaudhri M. Low dose naltrexone and lung cancer: a case report and discussion. Cureus. 2018;10(7):e2924.
- Cant R, Dalgleish AG, Allen RL. Naltrexone inhibits IL-6 and TNFα production in human immune cell subsets following stimulation with ligands for intracellular toll-like receptors. Front Immunol. 2017;8:809.
- Hutchinson MR, Zhang Y, Brown K, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur J Neurosci. 2008;28(1):20-29.
- Wang X, Zhang Y, Peng Y, et al. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br J Pharmacol. 2016;173(5):856-869.
- Lewis SS, Loram LC, Hutchinson MR, et al. (+)-naloxone, an opioid-inactive toll-like receptor 4 signaling inhibitor, reverses multiple models of chronic neuropathic pain in rats. J Pain. 2012;13(5):498-506.
- Theberge FR, Li X, Kambhampati S, et al. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry. 2013;73(8):729-737.
- Northcutt AL, Hutchinson MR, Wang X, et al. DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry. 2015;20(12):1525-1537.
- Hutchinson MR, Northcutt AL, Hiranita T, et al. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci. 2012;32(33):11187.
- McLaughlin PJ, Zagon IS. Duration of opioid receptor blockade determines biotherapeutic response. Biochem Pharmacol. 2015;97(3):236-246.
- Dermody SS, Wardell JD, Stoner SA, Hendershot CS. Predictors of daily adherence to naltrexone for alcohol use disorder treatment during a mobile health intervention. Ann Behav Med. 2018;52(9):787-797.
- Sullivan MA, Bisaga A, Pavlicova M, et al. A Randomized trial comparing extended-release injectable suspension and oral naltrexone, both combined with behavioral therapy, for the treatment of opioid use disorder. Am J Psychiatry. 2019;176(2):129-137.
Author acknowledgments: Ting-Hsuan Chiang, MD, Department of Anesthesiology & Perioperative Care, University of California Irvine, Irvine, CA. Kenneth Schmitt, BS, Department of Anesthesiology & Perioperative Care, University of California Irvine, Irvine, CA. Ariana Nelson, MD, Department of Anesthesiology & Perioperative Care, University of California Irvine, Irvine, CA.
Click Here to download the article PDF
