Urgent message: Throughout the COVID-19 pandemic, unvaccinated people have shown higher rates of morbidity and mortality in comparison with those who are fully immunized. While most vaccination adverse reactions are mild and self-resolving, it is important to consider the timeline of vaccinations to correlate possible adverse reactions.
Amanda dos Santos, MD and Michael Pallaci, DO, FACEP, FACOEP
INTRODUCTION
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic continues to affect all aspects of our society and its end seems illusory as we continue to have intermittent outbreaks. Most recent variants reinforced the importance of vaccination against coronavirus disease (COVID) as severe cases have almost exclusively affected the unimmunized. With over 12 billion doses administered worldwide,1 vaccines against COVID have proven to be safe and effective.
According to the Centers for Disease Control and Prevention, rare life-threatening reactions such as anaphylaxis have been reported in 5/1,000,000 injections, most often occurring in people with history of severe allergies; no deaths due to anaphylaxis have been reported, however.2 Although the Vaccine Adverse Event Reporting System (VAERS) has received death reports days to months after the vaccination, thorough investigation has failed to prove correlation.3 In comparison, over 6 million deaths have been directly linked to acute coronavirus infection.1
Nevertheless, as with any pharmaceutical treatment, mRNA vaccines have shown mild adverse reactions. Commonly reported side effects after the mRNA vaccines available in the United States—Pfizer-BioNTech BNT162b2 and Moderna mRNA-1273—include injection site pain, cutaneous reactions, generalized fatigue and weakness, myalgias, headache, chills, and fever. These symptoms tend to be minor and temporary, with a small fraction of patients requiring hospitalization (0.25% in an earlier study).2,4
Cutaneous reactions are commonly observed after viral infections and immunizations.5 Here, we present an uncommon case of bullous pemphigoid (BP) associated with the second dose of the Pfizer-BioNTech vaccine.
CASE REPORT
A 79-year-old man with history of insulin-dependent type II diabetes, end-stage renal disease on hemodialysis, hypertension, and coronary artery disease presented with a 4-week history of rash. It began on his right arm the day after receiving the second dose of the Pfizer-BioNTech vaccine, and then quickly became generalized. He described it as itchy but nontender “sores filled with water.” Beside the COVID-19 vaccine, he denied any new medications, triggers, or exposures and had never had a similar rash. He had seen a dermatologist for the rash and despite adherence to prescribed doxycycline, prednisone, niacinamide, hydroxyzine and topical triamcinolone for the preceding 4 weeks, the blisters continued to grow and spread, impairing his ability to perform basic activities such as dressing, bathing, sitting, or lying down.
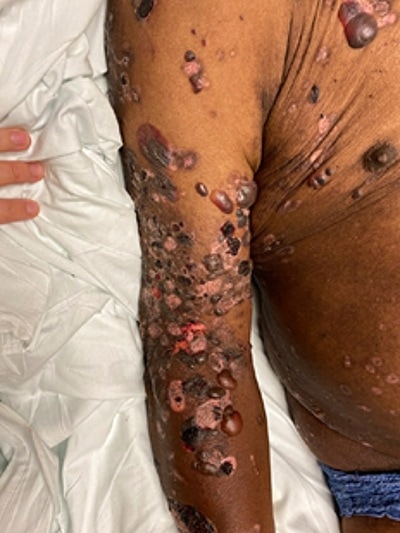
Physical exam revealed a chronically ill appearing gentleman with diffuse firm and flaccid bullae throughout his trunk, upper and lower extremities, head, face, feet but sparing the palms, soles, and mucous membranes (Figure 1).
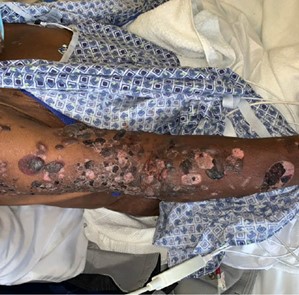
Blood work did not show leukocytosis or elevated inflammatory markers. He was treated with 10 mg IV dexamethasone and admitted for a rituximab infusion. After one infusion of 1000 mg of rituximab and he reported almost instantaneous symptomatic relief as the blisters began to subside (Figure 2).
A punch biopsy revealed pathological evidence of subepidermal blisters with eosinophils, immunoreactivity against C3 complement, and immunoglobulin IgG in a linear fashion along the basement membrane; thus, he was diagnosed with BP due to a drug eruption. He was discharged home on hospital day 2 with recommendation to continue prior treatments and outpatient follow-up.
However, the initial improvement was temporary and the blisters restarted days later. He underwent a second infusion of the monoclonal antibody 1 month later, and over the following 3 months the patient completed multiple courses of oral steroids, doxycycline, niacinamide, hydroxyzine, and triamcinolone ointment with significant improvement.
Unfortunately, 6 months after his initial symptoms (4 months after his first rituximab treatment) the patient died of refractory septic shock secondary to pneumonia.
DISCUSSION
The SARS-CoV-2 pandemic has most severely impacted the elderly and those with medical comorbidities, who also experienced more vaccine reactions, albeit at lower rates and severity than COVID itself.
BP is an autoimmune cutaneous reaction characterized by tense pruritic blisters secondary to hemidesmosomes destruction. The autoimmune dysregulation of T cells, IgG and IgE autoantibodies against hemidesmosome proteins in the epidermal-dermal junction leads to neutrophil chemotaxis and destruction of the basement membrane.6 Diagnosis is made by histologic evidence of eosinophilic spongiosis or subepidermal detachment, IgG, and/or C3 deposition along the basement membrane, and evidence of autoantibodies against basement membrane proteins (BP180 and/or BP230).Treatment usually involves high-dose topical and systemic steroids, as well as antibiotics and immunosuppressants for severe or refractory cases.6
Postimmunization BP has been reported after influenza, tetanus, diphtheria, hepatitis B, varicella-zoster, human papillomavirus, pertussis, poliomyelitis, rabies, Haemophilus influenzae B, typhoid, measles, pneumococcus, swine flu, and anthrax vaccines.5
The pathogenesis of the correlation of vaccines with BP is unknown. Immunization-induced pro-inflammatory cytokines and the release of proteolytic enzymes leading to hemidesmosome disruption,7 and immunological predisposition by means of CD25 deficiency or T helper cell dysfunction, have been postulated.7
To date, VAERS has received reports of 276 cases of BP following COVID vaccines.3 While many clinicians thought post-COVID vaccine BP to be one of the first reported cases, since the summer of 2021 several cases were published in the dermatology, immunology, and infectious diseases literature throughout the world.
In Malta, Young, et al reported a 68-year-old male who developed blisters 3 days after the first Pfizer BioNTech vaccine, which worsened after its second dose and resolved after 3 months of steroids.8
In Spain, Perez-Lopez, et al described a 78-year-old woman with blisters first noted 3 days after the first Comirnaty (Pfizer–BioNTech) vaccine that initially self-resolved, then restarted after the second dose and resolved after a short course of oral steroids.9
In Japan, Nakamura et al reported an 83‐year‐old woman with eczema (on topical steroids) who 3 days after the second dose of tozinameran (Pfizer–BioNTech) developed a diffuse BP rash that required oral steroids and high‐dose intravenous immunoglobulin therapy with gradual improvement.10
In Italy, there were two accounts after the first dose of the Comirnaty vaccine: Dell’Antonia et al described an 83‐year‐old man with mild pruritic blisters 1 week after the first dose that worsened after the second dose and resolved after 3 weeks of oral prednisone.11 Pauluzziand colleagues described the youngest case thus far, involving a 46-year-old man with noted BP blisters 15 days after his first injection who required 4 weeks of intramuscular methylprednisolone and oral azathioprine prior to improvement (he did not receive the second dose).12
In the United States, Kong, et al described a 66-year-old patient whose rash developed within 24 hours of the second dose of the Moderna vaccine after an uneventful first injection and who was also treated with high-dose oral steroids (outcome unknown).13 Khalid, et al likewise reported a severe case of blistery rash on a 62-year-old male 2 weeks after the first dose of the Moderna vaccine, which initially self-resolved but had a severe recurrence after the second dose requiring ICU admission (treatment and outcome not reported).14
There is also one report of BP following the carrier vaccine AstraZeneca from Morocco by Agharbi, et al in which a 77-year-old patient developed a diffuse pruritic bullous eruption 24 hours after the first injection and was treated with topical propionate of clobetasol 0.05% cream and doxycycline with improvement.15
CONCLUSION
Unlike most cases of vaccination reaction, this case highlights a rare severe immunogenic skin reaction that required multiple courses of treatment. Sadly, our patient who was already immunocompromised from his ESRD and diabetes expired 4 months after his presentation following rituximab infusions and multiple courses of steroids.
As the population continues to become infected with the coronavirus, we will likely be treating the cascade effects of COVID for years to come. Though many vaccine reactions are mild and can be managed without medical care, it is important to consider the timeline of immunizations and possible reactions, particularly in the chronically ill and elderly presenting with new symptoms.
Potential vaccine reactions are often benign and short-lived and vaccination against SARS-CoV-2 should continue to be encouraged by clinicians as it far outweighs the risks of the disease. Severe immunological reactions such as in this case are rare but should be accurately reported for scientific advancement and academic progress.
REFERENCES
- Johns Hopkins University & Medicine Coronavirus Resource Center. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/. Accessed January 11, 2022.
- U.S. COVID-19 vaccine product information. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/index.html. Accessed January 22, 2022.
- United States Department of Health and Human Services (DHHS), Public Health Service (PHS), Centers for Disease Control (CDC) / Food and Drug Administration (FDA), Vaccine Adverse Event Reporting System (VAERS) 1990 – 12/31/2021, CDC WONDER On-line Database. Available at: http://wonder.cdc.gov/vaers.html. Accessed January 11, 2022.
- Kadali RAK, Janagama R, Peruru S, et al. Side effects of BNT162b2 mRNA COVID-19 vaccine: a randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376-381. [Published online ahead of print April 15, 2021.]
- Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: a systematic review. J Am Acad Dermatol. 2021;S0190-9622(21)00899-9. [Published online ahead of print April 24, 2021.]
- Miyamoto D, Santi CG, Aoki V, et al. Bullous pemphigoid. An Bras Dermatol. 2019;94(2):133-146. [Published online ahead of print May 9, 2019.)
- Baroero L, Coppo P, Bertolino L, et al. Three case reports of post immunization and post viral bullous pemphigoid: looking for the right trigger. BMC Pediatrics. 2017;17(1):60.
- Young J, Mercieca L, Ceci M, et al. A case of bullous pemphigoid after the SARS-CoV-2 mRNA vaccine. J Eur Acad Dermatol Venereol. 2022;36(1):e13-e16.
- Pérez-López I, Moyano-Bueno D, Ruiz-Villaverde R. Bullous pemphigoid and COVID-19 vaccine. Bullous pemphigoid and COVID-19 vaccine. Med Clin (Barc). 2021;157(10):e333-e334.2021.05.005
- Nakamura K, Kosano M, Sakai Y, et al. Case of bullous pemphigoid following coronavirus disease 2019 vaccination. J Dermatol. 2021;48(12):e606-e607.
- Dell’Antonia M, Anedda S, Usai F, et a. Bullous pemphigoid triggered by COVID-19 vaccine: rapid resolution with corticosteroid therapy. Dermatol Ther. 2022;35(1):e15208.
- Pauluzzi M, Stinco G, Errichetti E. Bullous pemphigoid in a young male after COVID-19 mRNA vaccine: a report and brief literature review [published online ahead of print, December 20, 2021]. J Eur Acad Dermatol Venereol. 2021;10.1111/jdv.17891.
- Kong J, Cuevas-Castillo F, Nassar M, et al. Bullous drug eruption after second dose of mRNA-1273 (Moderna) COVID-19 vaccine: case report. J Infect Public Health. 2021;14(10):1392-1394.
- Khalid M, Lipka O, Becker C. Moderna COVID-19 vaccine induced skin rash. Vis J Emerg Med. 2021;25:101108.
- Agharbi F-Z, Eljazouly M, Basri G, et al. Bullous pemphigoid induced by the AstraZeneca COVID-19 vaccine [published online ahead of print, September 30, 2021]. Ann Dermatol Venereol. 2021;S0151-9638(21)00091-0.2021.07.008.
More COVID-19 Articles
- Time To Presentation For Acute Otitis Media During The COVID-19 Pandemic
- COVID-19: New Zealand’s Urgent Care Story


