Published on
Download the article PDF: United States Sexually Transmitted Infections A Comprehensive Overview And Relevance To Urgent Care Centers
Urgent Message: Patients frequently utilize urgent care centers for testing and treatment of sexually transmitted infections. All clinicians can be a first line defense to control the spread and combat the current epidemic.
Alexandra Faraj, PA-S; Nadesha Muniz, MS Ed, PA-C
Key Words: Sexually Transmitted Infections; Chlamydia; Gonorrhea; Syphilis; Expedited Partner Therapy; Urgent Care
Abstract
The rise in sexually transmitted infections (STIs) in the United States is a major public health concern. Patients frequently utilize urgent care centers for addressing STI-related complaints due to quick appointments, discreetness, and availability. All healthcare providers, especially those in urgent care centers, should be familiar with patient assessment, diagnostic tests, screening guidelines, and updated treatments for STIs.
Introduction
The rise in some sexually transmitted infections (STIs) and the increasing demand for screening has become a growing public health concern in the United States. In 2023, the United States Centers for Disease Control and Prevention (CDC) reported approximately 2.4 million cases of chlamydia, gonorrhea, and syphilis combined.[1] From 2022 to 2023, the number of reported chlamydia cases increased among men by 1.3%. The reported cases of syphilis (all stages) increased by 1.0%, and the number of unknown duration or latent syphilis increased by 12.8%.1 The 2023 STI surveillance report is in vast contrast to the CDC report in 2020-2021 showing decreased STIs, which may be attributed to the restrictions in place during the global COVID-19 pandemic that essentially limited patients’ access to healthcare and testing services for non-emergent illnesses.[2] As the number of patients seeking treatment for STI complaints increases, urgent care centers have become the frontline for addressing these concerns.
Sexually Transmitted Infections At A Glance
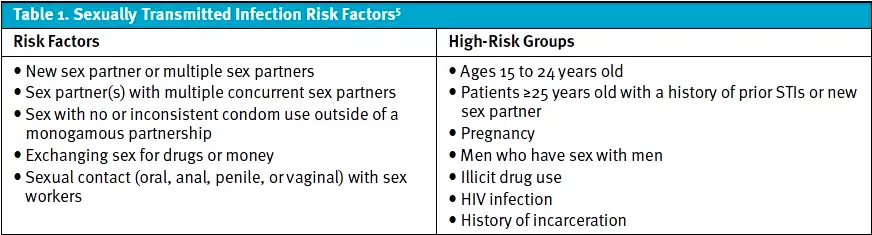
In 2023, chlamydia, gonorrhea, and syphilis were the most treated STIs in the United States.1 Chlamydia and gonorrhea infections are often asymptomatic in both sexes, however, common symptoms include vaginal discharge in females and urethritis in males. Females are more likely to present with atypical symptoms including vaginal pruritis, intermenstrual bleeding, or menorrhagia.[3],[4] The most common sites for inoculation are the urogenital tract, but other sites such as the oropharynx, rectum, and eye in both sexes may be involved.4 Young sexually active individuals or adults in high-risk groups and specific behavioral risk factors (Table 1) are associated with increased risk of STIs and infection-related morbidity.[5]
Chlamydia
Chlamydia is the number 1 reported STI in the United States with over 1.6 million reported cases in 2023. Young women ages 20 to 24 have the highest rate of chlamydia.1 Chlamydia is caused by Chlamydia trachomatis, an obligate intracellular Gram-negative bacterium with a distinct life cycle that contributes to its survival in hosts and ongoing transmission.[6] Chlamydia has an incubation period of 5 to 14 days for symptomatic infections and an unknown length of time for asymptomatic infections. Over 70% of urogenital infections and 90% of rectal and pharyngeal chlamydia infections are asymptomatic.4 Symptomatic chlamydia infections have clinical manifestations including urethritis (dysuria or urethral discharge), cervicitis (mucopurulent discharge), epididymitis (fever, testicular pain), and pelvic inflammatory disease (PID).4
Gonorrhea
The CDC reported over 600,000 cases of gonorrhea in 2023.1 Gonorrhea is caused by the Neisseria gonorrhoeae (N. gonorrhoeae) bacterium, a Gram-negative diplococcal aerobe. N. gonorrhoeae has physical and genetic properties that can quickly develop antibiotic resistance.[7] Following transmission, colonization, and an incubation period of about 2-8 days, human hosts may develop symptoms of an active infection. Ninety percent of symptomatic men with urogenital gonorrhea have urethritis (urethral discharge and dysuria).[8] However, up to 86% of urogenital gonorrhea infections in men and 92% of urogenital gonorrhea in women may be asymptomatic.4,[9] Without significant symptomatic distinction, the CDC recommendation is to test concurrently for chlamydia and gonorrhea infections and to treat for both while waiting for results of lab testing.
Long-Term Effects of Untreated Chlamydia and Gonorrhea
Although many patients are asymptomatic, they are still at risk for chronic sequelae from untreated infections and can transmit the infection to others. The long-term effects of untreated STIs may cause irreversible damage to the urogenital system or affect other organ systems.[10]
In women, pelvic inflammatory disease (PID) is among the most concerning complications of a chlamydia or gonorrhea infection. PID is an acute or subacute infection of the upper genital tract (uterus, fallopian tubes, or ovaries). Despite its typical origin stemming from an untreated chlamydia or gonorrhea infection, PID is considered a separate STI itself. PID is a clinical diagnosis, and providers should have a low threshold for diagnosis and treatment.10
The CDC criteria that support the diagnosis of PID include:[11]
- Sexually active young women and other women at risk for STI presenting with unexplained pelvic or lower abdominal pain and have1 of the 3 minimum clinical criteria present on pelvic examination: cervical motion tenderness; uterine tenderness; or adnexal tenderness on exam
- Additionally, 1 of the following criteria can support a PID diagnosis:
- Fever with an oral temperature >101°F or (>38.3°C)
- Mucopurulent cervical discharge or cervical friability (easily bleeds)
- Multiple white blood cells in vaginal fluid visualized on saline microscopy
- Increased erythrocyte sedimentation rate (ESR)
- Increased C-reactive protein (CRP)
- Positive laboratory documentation of N. gonorrhoeae or C. trachomatis
Common complications of PID include:10
- Endometritis: acute localized inflammation of the endometrium (uterine lining)
- Salpingitis: acute inflammation of 1 or both fallopian tubes
- Tubo-ovarian abscess: a pocket of pus in the fallopian tube that extends into the ovaries
- Pelvic peritonitis: inflammation and infection of the peritoneum
- Infertility: difficulty getting pregnant
- Ectopic pregnancy: pregnancy outside of the uterus, typically in the fallopian tube or ovary
Systemic complications of PID include:
- Perihepatitis (Fitz-Hugh-Curtis syndrome): inflammation of the liver capsule and peritoneal surface, which excludes the liver tissue, and leads to adhesion formation[12]
- Reactive arthritis: inflammation of the joints. For patients who present with a triad of conjunctivitis, urethritis, and arthritis, the differential diagnosis should include STIs12,[13]
Syphilis
Treponema pallidum (T. pallidum), commonly known as syphilis, is theorized to have existed as far back as the late 1400s. Recently, syphilis infections have increased, with approximately 209,000 cases reported in 2023.1 T. pallidum is a spirochete, and humans are the only known hosts. With a long incubation period of 3-4 weeks and a slow replication rate of approximately 30 hours, there is ample room to screen and detect syphilis. The bacterium is known for its stealth, with physical characteristics and properties that make it difficult to identify for both immune systems and routine microscopy lab testing.[14]
After an initial inoculation, syphilis progresses in 4 infectious stages if untreated. In contrast to chlamydia and gonorrhea, symptoms of syphilis are significantly more distinct in early infection. A single, painless chancre is 1 of the initial clinical manifestations of stage 1 syphilis infection. A chancre is a local immune response that manifests as a painless ulcer, signifying the initial site where the bacterium entered host tissue. It starts as a papule and progresses to a round, indurated ulcer. Unfortunately, unless the point of inoculation is external, many patients progress through this stage unaware of an active infection. Shortly after primary syphilis, patients develop secondary and ultimately tertiary syphilis infection caused by bacteria dissemination, during which it is challenging to identify (thus why it is known as “the great imitator”). Syphilis can affect almost every organ upon dissemination, from a macular rash (secondary syphilis) and cardiovascular involvement (tertiary syphilis) to stroke and dementia (neurosyphilis), which may become apparent as many as 30 years after the initial infection.14 Of note, syphilis can be fatal in rare cases. In 2023, the CDC reported 279 congenital syphilis stillbirths and neonatal/infant deaths.1 As such, diagnosis and treatment of pregnant women is critical to prevent neonatal infections.
STI Diagnosis and Screening Tests
Screening is among the most crucial steps to combat the STI epidemic.[15] Previously, local health department specialty STI clinics were the primary location for screening and treatment services. More recently, this responsibility has expanded beyond STI clinics due to a lack of government funding and subsequent closures.[16] With this, the demand for STI-related services has increased in primary care, urgent care, family planning clinics, and emergency departments. According to the CDC, in 2018 non-STI clinics reported almost 80% of all STI cases. As such, a multispecialty approach is necessary to help combat the STI epidemic, with all providers playing a crucial role in diagnosing and treating STIs.[17]
The framework of STI screening and testing is based heavily on taking an accurate sexual history. According to the American Academy of Family Physicians (AAFP), the best practice is to obtain an accurate sexual history with the “5 P’s of Sexual Health.”[18] The 5 P’s represent the core information that is pertinent to a patient’s sexual health and significantly contributes to STI screening. The 5 P’s provide detailed information about:
- Patient’s partners: ask about current sexual partners of any gender or type of sex
- Practices (sexual): ask specific questions about type of sex (vaginal, anal, or oral)
- Protection from STIs: ask about prevention of STIs, specifying condom use
- Past history of STIs: include questions about past testing and past diagnosis of STIs of the patient and partners
- Pregnancy prevention: include questions about plans for children and use of contraceptives; offer options for preventing pregnancy if patient desires
An accurate sexual history helps providers identify patients at higher risk for STIs. The United States Preventive Services Task Force (USPSTF) and the CDC suggest at least annual screening of all sexually active women age 24 or younger and women 25 and older (including pregnant women) who are at increased risk of chlamydia and gonorrhea infection. Patients who are not sexually active or in a monogamous relationship are not at increased risk.18,[19] The CDC additionally recommends screening all pregnant women during their first obstetric visit for syphilis.18
Chlamydia and Gonorrhea Testing
Nucleic acid amplification tests (NAATs) for Chlamydia trachomatis and Neisseria gonorrhoeae organisms are the gold standard for diagnosis due to their high sensitivity and specificity. NAATs can be performed on urine specimens; female endocervical and vaginal specimens; and male urethral, rectal, and pharyngeal specimens.15,[20] A vaginal swab (including a self-collected swab) specimen is preferred in women, and first-catch urine specimen is preferred in men.19 In addition to NAAT, culture should remain an option for symptomatic individuals, especially if there are concerns of antibiotic resistance or in legal cases.20
Syphilis Testing
Typically, diagnosis of syphilis requires 2 laboratory serologic tests: a nontreponemal (lipoidal antigen) test (venereal disease research laboratory or rapid plasma reagin test); and a treponemal test (T. pallidum passive particle agglutination) assay.4 Primary lesions concerning for chancre can be swabbed and sent to lab for identification via polymerase chain reaction (PCR).[21]
Treatment Protocols
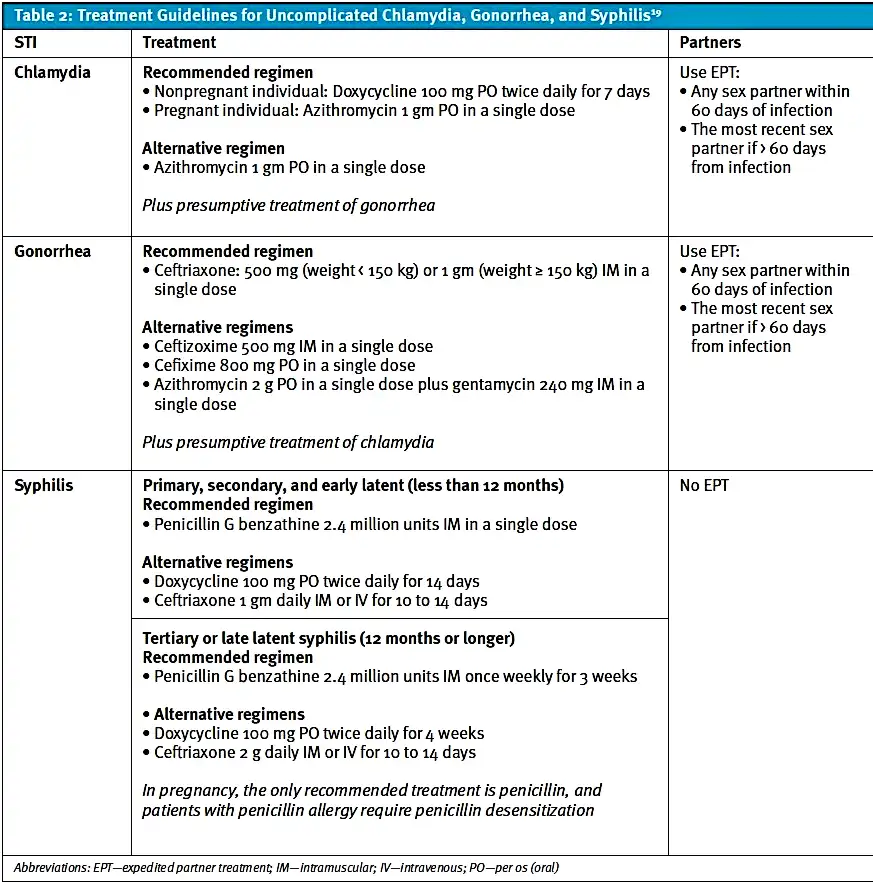
Pharmacologic treatment guidelines for STIs are concise and straightforward. For a suspected or confirmed diagnosis of an STI, the patient should be provided prescription medication and education about sexual health and protection. Additionally, the provider may offer expedited partner treatment (EPT). In 2021, the CDC updated its guidelines for the treatment of chlamydia, gonorrhea, and syphilis (Table 2).19
Table 2: Treatment Guidelines for Uncomplicated Chlamydia, Gonorrhea, and Syphilis19
Follow-Up
To combat high rates of transmission, patients should refrain from sexual activity until treatment is completed, and providers should discuss the use of protection to decrease the risk of contracting future STIs. As such, providers may reference the “Five P’s of Sexual Health” to guide this conversation. Patients should not follow up for repeat testing until at least 3 months following treatment of urogenital infection.19 The treatment protocols for STIs are concrete and practical, allowing our public healthcare system to reduce the transmission rate.
EPT has enabled providers to make impact on slowing the spread of STIs. EPT was enacted in 2009, which gave providers the ability to treat sexual partners of patients diagnosed with chlamydia or gonorrhea without the need for evaluation in person.[22] This public health strategy not only gives providers better access to patients’ sexual partners for treatment, but it also allows those partners to remain somewhat anonymous, increasing their likelihood to undergo treatment. However, EPT is often underutilized by providers and patients. According to the CDC, EPT is legal in many states and can be a valuable option for patients especially when packaged oral medication is offered and accompanied by educational materials on use and potential allergies. Providers are encouraged to visit the CDC website to obtain updated information for their state.[23]
Urgent Care’s Role
A survey found that more than 60% of STI specialty clinics report budget cuts while the demand for STI-related healthcare continues.16 Urgent care centers are attractive for patients looking for screening, testing, or treatment of an STI quickly and discreetly.[24] Most urgent cares can perform STI testing with a simple urine sample, which is collected and sent to a lab for PCR testing with a 3–5-day turnaround time. However, some offer NAAT point-of-care testing, which entails rapid STI testing performed in as little as 30 minutes.[25] Additionally, patients may utilize an at-home test for common STIs, thus presenting to urgent care centers for treatment following an at-home positive result.
Takeaway Points
- Chlamydia, gonorrhea, and syphilis infections are on the rise.
- Young sexually active patients or older patients with high-risk behaviors are at higher risk for acquiring STIs.
- Asymptomatic or atypical presentations of chlamydia lead to an increased risk of delayed diagnosis and potential complications.
- USPSTF and CDC have released updated STI screening, diagnosis, and treatment guidelines addressing resistance, allergies, specific patient considerations, and recommendations for EPT.
- Urgent care centers are one of the most promising settings for controlling the spread and combating the STI epidemic.
Manuscript submitted May 5, 2025; accepted September 16, 2025.
References
- [1]. Centers for Disease Control and Prevention. National overview of STIs in 2023. Published November 14, 2024. Accessed August 18, 2025. https://www.cdc.gov/sti-statistics/annual/summary
- [2]. Pagaoa M, Grey J, Torrone E, Kreisel K, Stenger M, Weinstock H. Trends in nationally notifiable sexually transmitted disease case reports during the US COVID-19 pandemic, January to December 2020. Sex Transm Dis. 2021;48(10):798-804. doi:10.1097/OLQ.0000000000001506
- [3]. Cantor A, Dana T, Griffin JC, et al. Screening for chlamydial and gonococcal infections: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326(10):957-966. doi:10.1001/jama.2021.10577
- [4]. Tuddenham S, Hamill MM, Ghanem KG. Diagnosis and treatment of sexually transmitted infections: a review. JAMA. 2022;327:161-172. doi:10.1001/jama.2021.23487
- [5]. Centers for Disease Control and Prevention. Sexually transmitted infections treatment guidelines, 2021: STI and HIV infection risk assessment. Published July 22, 2021. Accessed August 18, 2025. https://www.cdc.gov/std/treatment-guidelines/default.htm
- [6]. Witkin SS, Minis E, Athanasiou A, Leizer J, Linhares IM. Chlamydia trachomatis: the persistent pathogen. Clin Vaccine Immunol. 2017;24(10):e00203-17. doi:10.1128/CVI.00203-17
- [7]. Criss AK, Seifert HS. A bacterial siren song: intimate interactions between Neisseria and neutrophils. Nat Rev Microbiol. 2012;10(3):178-190. doi:10.1038/nrmicro2713
- [8]. Unemo M, Seifert HS, Hook EW 3rd, Hawkes S, Ndowa F, Dillon JAR. Gonorrhoea. Nat Rev Dis Primers. 2019;5(1):79. doi:10.1038/s41572-019-0128-6
- [9]. Centers for Disease Control and Prevention. Sexually transmitted infections treatment guidelines, 2021: Gonococcal infections among adolescents and adults. Accessed August 18, 2025. https://www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm
- [10]. Centers for Disease Control and Prevention. Sexually transmitted infections treatment guidelines, 2021: Pelvic inflammatory disease (PID). Accessed August 18, 2025. https://www.cdc.gov/std/treatment-guidelines/pid.htm
- [11]. He W, Jin Y, Zhu H, Zheng Y, Qian J. Effect of Chlamydia trachomatis on adverse pregnancy outcomes: a meta-analysis. Arch Gynecol Obstet. 2020;302(3):553-567. doi:10.1007/s00404-020-05664-6
- [12]. Aitken-Saavedra J, Maturana-Ramirez A, Fernández Moraga J, Doro Dias V, Galdino-Santos L, Pineda Flores D. Reactive arthritis: images. Dermatol Online J. 2021;27(7). doi:10.5070/D327754373
- [13]. Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382(9):845-854. doi:10.1056/NEJMra1901593
- [14]. Greydanus DE, Cabral MD, Patel DR. Pelvic inflammatory disease in the adolescent and young adult: an update. Dis Mon. 2022;68(3):101287. doi:10.1016/j.disamonth.2021.101287
- [15]. Cantor A, Dana T, Griffin JC, et al. Screening for chlamydial and gonococcal infections: a systematic review update for the US Preventive Services Task Force (Evidence Synthesis No. 206). Agency for Healthcare Research and Quality; 2021. AHRQ Publication No. 21-05275-EF-1.
- [16]. Luk C, Palar K, Ludovic J, et al. Staffing and Budget Levels at Local Sexually Transmitted Disease Programs by County-Level Sociodemographic Characteristics During COVID-19. Sex Transm Dis. 2025;52(8):e37-e40. doi:10.1097/OLQ.0000000000002152
- [17]. Barrow RY, Ahmed F, Bolan GA, Workowski KA. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Recomm Rep. 2020;68(RR-5):1-20. doi:10.15585/mmwr.rr6805a1
- [18]. Yonke N, Aragón M, Phillips JK. Chlamydial and gonococcal infections: screening, diagnosis, and treatment. Am Fam Physician. 2022;105(4):388-396.
- [19]. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1-187. doi:10.15585/mmwr.rr7004a1
- [20]. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
- [21]. Theel ES, Katz SS, Pillay A. Molecular and direct detection tests for Treponema pallidum subspecies pallidum: a review of the literature, 1964–2017. Clin Infect Dis. 2020;71(Suppl 1):S4-S12. doi:10.1093/cid/ciaa176
- [22]. Jackson KJ, Pickett ML. Expedited partner therapy review. Pediatr Emerg Care. 2024;40(11):828-832. doi:10.1097/PEC.0000000000003275
- [23]. Centers for Disease Control and Prevention. Sexually Transmitted Infections Treatment Guidelines, 2021: Expedited Partner Therapy. Published July 22, 2021. Accessed August 18, 2025. https://www.cdc.gov/std/treatment-guidelines/ep-tx.htm
- [24]. Ayers A. The business case for STI testing in urgent care centers. J Urgent Care Med. 2024;18(10):39-42.
- [25]. Fisk KM, Derouin A, Holm G, Hicks L. Getting it right: the impact of point-of-care testing for gonorrhea and chlamydia in the urgent care setting. J Nurse Pract. 2020;16(5):388-393. doi:10.1016/j.nurpra.2020.01.006
Author Affiliations: Alexandra Faraj, PA-S, St. John’s University. Nadesha Muniz, MS Ed, PA-C, St. John’s University. Authors have no relevant financial relationships with any ineligible companies.
Read More
- Postpartum Presentations: When Risk Arises After Delivery – Vaginal Bleeding and Discharge
- The Urgent Need for STI Testing in Urgent Care Centers
