Urgent message: With the increasing number of drugs on the market, patients are more and more likely to be taking multiple medications. Urgent care providers need to be alert for potential interactions when changing or adding to a patient’s drug therapy.
MAYA HECK, MS1, and JOHN SHUFELDT, MD, JD, MBA, FACEP
Consider This Patient Scenario:
A 28-year-old woman who says she is a traveling nurse currently on an assignment at a nearby hospital presents to an urgent care clinic with a complaint of frequency, urgency and burning on urination. She is going on a ski trip and wants to ensure that she clears up what she believes is a urinary tract infection (UTI) before leaving on vacation. The patient denies vomiting but complains of intermittent nausea. She denies flank pain, vaginal discharge, and the possibility of pregnancy. Interestingly, the woman does tell the provider that she was on birth control pills (BCPs) but developed a blood clot in her thigh and then a pulmonary embolism (PE) after flying cross country. She has been on warfarin for the past 3 months and her last International Normalized Ratio (INR) (yesterday) was 2.4. Also noteworthy is that the woman has had a significant reaction to penicillin-based antibiotics in the past.
The urgent care provider orders a urinalysis (UA) and urine chorionic gonadotrophin (UCG) test. On examination, the patient’s vital signs are as follows:
- BP 120/72 P 88
- R 16
- T 39.2
- Pain Scale 4/10
The woman’s exam is unremarkable, other than some slight suprapubic tenderness. Her urine dipstick comes back with 4+ leukocytes and is nitrite positive and her UCG is negative.
She is appropriately diagnosed with a UTI. Noting the patient’s allergy to penicillin, the provider prescribes trimethoprim and sulfamethoxazole (TMP-SMX) and pyridium and discharges the patient home.
A few days later, sadly, the patient was flown into a trauma center in Denver unresponsive after a seemingly minor head injury, which occurred during a snowboarding accident while she was wearing a helmet. On admission, the woman’s Glasgow Coma Scale score was 3 and her INR was 8.2. Computed tomography scan revealed a massive epidural hematoma causing herniation. Her family removed her from life support after donating her organs.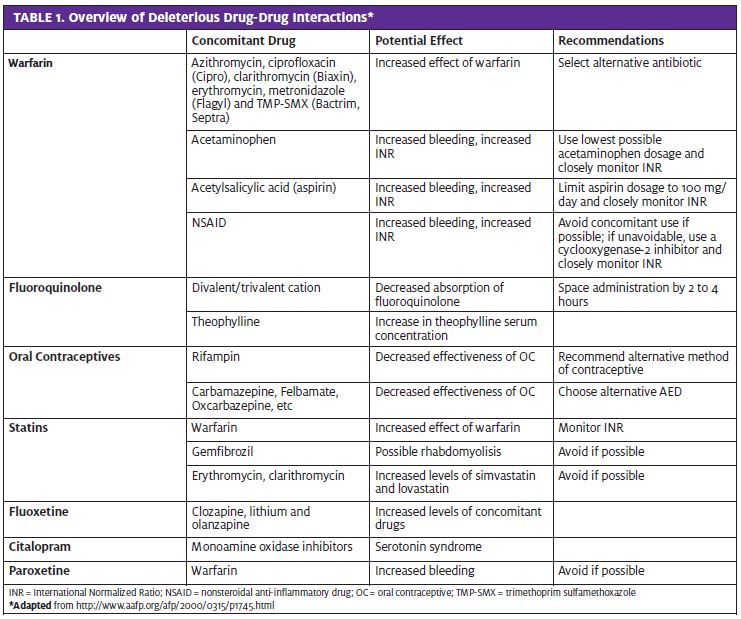
The Rise of Drug-Drug Interactions
In 2012, the FDA approved 35 new medications 1, and inter- actions between medications have been increasingly reported.2 As patients’ medication lists become longer and the use of multiple drug therapies becomes more frequent, it is increasingly important for the provider to consider drug interactions and question the safety of drug regimens. Among other risk factors, the frequency of drug-drug interactions increases most significantly with the number of medications in use. Adverse drug events, including drug-drug interactions, account for 19% of all hospital injuries 3 and most involve commonly-used medications. By underscoring the potential for drug interactions with warfarin, as illustrated by the patient scenario above, this article highlights the importance of recognizing drug-drug interactions and considering the factors that increase their risk.
Drug Interactions with Warfarin
Warfarin is the anticoagulant most widely prescribed in North America4 and interactions leading to over-anticoagulation, under-coagulation, or increased bleeding are well described for an increasingly large number of drugs taken concomitantly. Warfarin and other vitamin K antagonists (acenocoumarol, phenprocoumon, fluindione) are prescribed as oral anticoagulants for thrombosis prophylaxis in many clinical settings. This class of drugs act by inhibiting the synthesis of vitamin K, a vitamin necessary for activation of coagulation factors. Warfarin is known to have a narrow therapeutic range and dose response and result- ant INR vary greatly between individuals. Especially notable are the interactions between warfarin and antibiotics, acetaminophen, aspirin, and nonsteroidal anti-inflammatory drugs (NSAIDs) (Table 1).
For urgent care providers, obtaining a thorough history is the best way to prevent poor outcomes due to unforeseen drug-drug interactions. It is important to review medication lists and consider possible drug-drug interactions before prescribing new medications to patients using warfarin.
Antibiotics
By reducing vitamin K levels in the gut flora, antibiotics can heighten the effects produced by warfarin, leading to over-anticoagulation.5 Another mechanism recognized for increased bleeding with warfarin is inhibition of its metabolism. Drugs that have been found to inhibit warfarin metabolism include azithromycin, ciprofloxacin (Cipro), clarithromycin (Biaxin), erythromycin, metronidazole (Flagyl) and TMP-SMX (Bactrim, Septra).2
Upon its release in 1993, azithromycin was originally believed to be free of interaction with warfarin. However, in 2009, the FDA revised its label on the pack- age insert to read, “Although, in a study of 22 healthy men, a 5-day course of azithromycin did not affect the prothrombin time from a subsequently administered dose of warfarin, spontaneous post-marketing reports suggest that concomitant administration of azithromycin may potentiate the effects of oral anticoagulants. Prothrombin times should be carefully monitored while patients are receiving azithromycin and oral anticoagulants concomitantly.”6
In a population-based cohort study of patients using acenocoumarol or phenprocoumon, several antibiotics strongly increased the risk of over-anticoagulation; 351 of the 1,124 patients in the cohort developed an INR of
- In the study, TMP-SMX was associated with the greatest risk of over-anticoagulation.7 Given these findings, urgent care providers should select an alternative antibiotic that has not been implicated in over-anticoagulation for patients who are taking warfarin.
Acetaminophen
Acetaminophen is the most frequently ingested medication in the United States2 and it has been recognized as another cause of over-anticoagulation. In one study, high doses of acetaminophen (9100 mg/week or just 3 extra- strength tablets per day) were found to be associated with a 10-fold increased risk of having an INR > 6.0.8 In a case report, acetaminophen was found to enhance the effect of warfarin but the elevation may only be apparent after a few days of acetaminophen therapy.9
Nonsteroidal Anti-Inflammatory Drugs
Concomitant use of NSAIDs and warfarin increases risk of bleeding and should be avoided. However, in a study assessing rates of hospitalization for gastrointestinal bleeding, the selective cyclooxygenase-2 (COX-2) inhibitors celecoxib (Celebrex) and rofecoxib (Vioxx) were prefer- able to nonselective NSAIDs in patients who required NSAIDs.10 COX-2 inhibitors have reduced antiplatelet properties compared with nonselective NSAIDs.
Also noteworthy is that the particular need to avoid use of nonselective NSAIDs in patients who are at increased risk of NSAID gastropathy. Factors including age > 65, his- tory of peptic ulcer disease, exposure to systemic steroid therapy, heavy smoking, or high usage of NSAIDs are red flags for heightened risk of gastropathy.2
Counseling patients about the risks of bleeding associated with concomitant therapy with warfarin and NSAIDs and close monitoring of INR values are important to minimize risk of bleeding.
Monitoring INR Values
According to UpToDate, the guidelines for returning INR values to the patient’s normal range are as follows:
- INR <5.0 without bleeding: If the INR is above normal range but less than 5.0 with no significant bleeding, it is recommended that the next dosage of warfarin be omitted or the maintenance dose be 11
- INR 0-9.0 without bleeding: If there is no significant bleeding, there is approximately a 1% risk of a major hemorrhage occurring in the next 30 days. Two of the options for reducing INR values are:
- Stopping warfarin
Stopping warfarin temporarily and adding a dose of 1 to 2.5 mg of oral vitamin K. This is a faster approach to correct excessive anticoagulation.11
- INR >9.0 without bleeding: If there is no significant bleeding with these INR values, warfarin should be omitted and 2.5 to 5 mg of oral vitamin K should be INR values should be closely monitored for 24 to 48 hours and vitamin K treatment should be repeated as necessary.11
- Elevated INR with minimal bleeding: Although there are no precise guidelines for this situation, the deci sion is based upon clinical judgment, INR level, and current extent/risk of worsening of the Providers may choose to follow treatment plans for INR >9.0 without bleeding, or opt for more emergent care depending on the severity of the case.11
Drug Interactions with Antibiotics
Fluoroquinolones
Antibiotics can interact with other drugs via multiple mechanisms; this article highlights significant interac- tions involving the fluoroquinolone class of antibiotics. Agents containing divalent cations and trivalent cations can reduce absorption of fluoroquinolones through creation of insoluble complexes in the gut, which results in failure of treatment. Cations are present in many medications and over-the-counter products, and it has been found that absorption of fluoroquinolones is reduced by 60% to 70% when they are taken concomitant with products containing divalent or trivalent cations.2
The divalent cation calcium is present in Caltrate, Citracal, Os-Cal, PhosLo, Titralac, and Tums, whereas the divalent cation magnesium is found in Almora, Citrate of Magnesia, Mag-Ox 400, Milk of Magnesia, Slow-Mag, and UroMag. Aluminum and ferrous sulfate are trivalent cations that can be found in Alu-Cap, AlternaGel, Amphojel, Basalje and Feosol, Fergon, Niferex, Nu-Iron, and Slow Fe, respectively.2
Ciprofloxacin, enoxacin, lomefloxacin, norfloxacin, and ofloxacin are affected by divalent and trivalent cations found in antacids. Sucralfate, containing the trivalent cation aluminum, has been found to inhibit absorption of several quinolones, including ciprofloxacin and norfloxacin.12
It is important to advise patients to stop taking products containing divalent or trivalent cations until their fluoroquinolone therapy has ceased.2 It has been shown that inhibition of absorption can be avoided by administering the antibiotic at least 2 hours before or 6 hours after the cation.12
Theophylline
Quinolones inhibit metabolism of theophylline, which results in an increase in theophylline serum concentrations and subsequent reactions, leading to tachycardia, nausea, and seizures.12 Quinolone antibiotics exhibit a wide range of inhibition: Enoxacin can result in a 65% reduction of theophylline clearance whereas ciprofloxacin inhibits by about 30%. Norfloxacin, ofloxacin, and lomefloxacin have been found to produce only a minimal effect.12
Patients with the upper normal limits of theophylline serum concentrations are more likely to develop these types of effects.12 This is an important factor to consider when determining whether the addition of a quinolone is advisable. Note: It can take 2 to 3 days after the combination of quinolone and theophylline for the effects to become pronounced, therefore, patients should be monitored for signs and symptoms of toxicity.
Drug Interactions with Oral Contraceptives
Antibiotics
Clinical studies have not been demonstrative of a consistent effect of antibiotics on the decreased effectiveness of oral contraceptives (OCs), and concomitant use is debatable. Given the low frequency of this drug-drug interaction, it is difficult to differentiate a pregnancy due to the antibiotic from the expected failure rate.2 Rifampin can impair the effectiveness of OCs because of its ability to increase activity of hepatic enzymes, which are involved in estrogen metabolism.2 Concomitant use of rifampin and OCs can lead to breakthrough bleeding and potentially an increased risk of pregnancy. Although pharmacokinetic studies of other antibiotics such as tetracycline and penicillin derivatives have not shown any systematic interactions with OCs, individual patients have been found to have decreased plasma concentrations of ethinyl estradiol. It is theorized that any interaction between OCs and antibiotics may involve ethinyl estradiol.
An analysis of 167 articles on drug interactions between antibiotics and OCs resulting in contraceptive failure found that approximately 20% of women reporting to family planning or abortion clinics were taking antibiotics and OCs concomitantly.13 However, a retrospective study of 365 patients who were co-administered OCs and an antibiotic showed only a small but insignificant increase in risk of pregnancy.2
According to the American College of Obstetricians and Gynecologists Committee Opinion on medical eligibility criteria for contraceptive use, there is no restriction on use of OCs with broad spectrum antibiotics, antifiungals, or antiparasitics. However, with regards to rifampicin or rifabutin therapy, the risk of pregnancy was found to outweigh the advantages of using OCs as a birth control method.14
Take home point: Concomitant use of broad spectrum antibiotics and OCs is considered to be a safe practice. However, urgent care providers may want to encourage patients on OCs to use back-up contraception during rifampicin therapy.
Antiepileptic Drugs
Prescription of OCs to women with epilepsy is fairly common, despite the knowledge that concomitant use may reduce the pill’s efficacy.15 Antiepileptic drugs (AEDs) that have been shown to induce metabolism of the steroidal components of the pill include carbamazepine, felbamate, oxcarbazepine, lamotrigine, phe- nobarbital, phenytoin, primidone, and topiramate.
However, the magnitude of interaction depends on the dosage of the AED. For example, topiramate does not affect serum norethisterone and ethinyl estradiol levels at dosages up to 100 mg per day but decreases serum norethisterone consistently at higher doses.15 Likewise, carbamazepine 600 mg per day reduces serum levels by 50%. Gabapentin, levetirac- etam, pregabalin, tiagabine, valproate, vigabatrin, and zonisamide have not been reported to interact with steroid OCs.15
Take home point: the best way to avoid complications with this drug-drug interaction is to choose an AED that is not known to interact with OCs.
Drug Interactions With ‘Statins’
Statins are effective in treatment of hypercholesterolemia because of their ability to reduce low-density lipoprotein cholesterol levels. However, there are many drugs that create adverse reactions when taken concomitant with statins; generally, the risk of drug-drug interactions is dose- dependent. Because of the likelihood that patients receiving statin therapy are elderly and may be on multiple other drugs for comorbid conditions such as heart disease or hypertension, it is especially important to be aware of statin drug interactions in these situations.
Drugs that raise statin concentrations in the blood and can increase risk of adverse interactions such as myopathy include immunosuppressant drugs, macrolides, fibrates, protease inhibitors, azole antifungals, warfarin, and digoxin.16 For example, one study reported myopathy incidence of 2%, 5% and 28% in patients receiving lovastatin therapy with niacin, gemfibrozil and cyclosporine plus gemfibrozil, respectively.16 It is important to be aware of CYP3A4 inhibitors when considering co-administration; a systematic review of statin safety including data from 20 randomized controlled trials showed that 60% of cases of rhabdomyolysis in patients receiving simvastatin, lovastatin or atorvastatin involved co-administration of CYP3A4 inhibitors, and 19% involved co-administration of fibrates.16
General Interactions
Verapamil and diltiazem have been shown to increase simvastatin plasma concentration up to fourfold, and diltiazem was also found to have the same effect with concomitant lovastatin therapy.16 Statin drug-drug interactions with warfarin are well documented, and an elevated INR is a concern with warfarin and various statins (fluvastatin, lovastatin, and simvastatin). Antibiotics that are CYP3A4 inhibitors, such as erythromycin and clarithromycin, have been reported to increase plasma concentration of simvastatin and lovastatin. Conversely, the antibiotic rifampicin has been demonstrated to decrease plasma levels of statins including atorvastatin, simvastatin, pravastatin, and fluvastatin.16 Azole antifungal drugs, such as itraconazole and etoconazole, have been shown to increase the availability of atorvastatin, simvastatin, lovastatin, and rosuvastatin.16
Fibrates
Of special importance is the recognition of fibratestatin interactions. Gemfibrozil has been shown to interact with atorvastatin, simvastatin, lovastatin, pravastatin, and rosuvastatin. However, bezafibrate, clofibrate and fenofibrate have also been implicated in cases of rhabdomyolysis when combined with statins. Because of the well-documented dangerous interactions with fibrate, statin-fibrate combination therapy should be reserved for patients with severe hyperlipidemia.16 In addition, it is also important to consider other factors, such as age, gender, diabetes, and hypothyroidism that may make a patient more suscetible to statin-induced myopathy.
Drug-drug interactions with statins are extensive, and only a few examples have been highlighted in this review. Take care in co-administering statins with the drugs mentioned above: immunosuppressant drugs, macrolides, fibrates, protease inhibitors, azole antifungals, war- farin, and digoxin.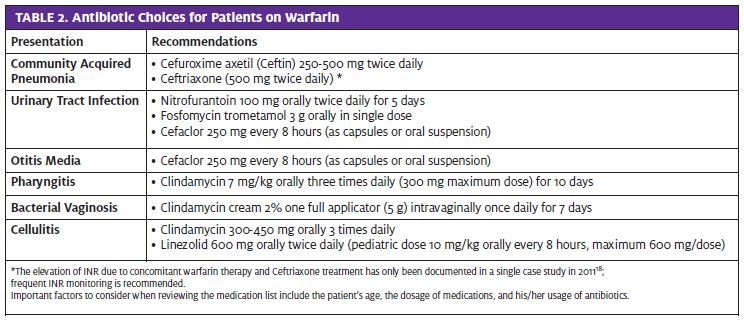
Interactions With the ‘New Generation’ of SSRIs
Drug interactions with tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) have been well documented. Since the advent of selective serotonin reuptake inhibitors (SSRIs), newer classes of antidepres- sants have been widely adopted due to their relative safety compared with previous antidepressants. Even though SSRIs are reportedly safer than the “first generation” of antidepressants, drug-drug interactions are still an issue because of metabolism by the cytochrome 450 system in the liver. The concomitant use of SSRIs and drugs that are also metabolized by this system can lead to increased serum levels of these drugs. This article will highlight the recently documented interactions with the “new generation” of SSRIs, including fluoxetine, citalopram, and paroxetine.
Fluoxetine
Fluoxetine (FLU) has a long half-life (1-4 days) and, for that reason, it has a high risk of interacting with other drugs. Plasma levels can be significant even weeks after discontinuation of FLU therapy, so care should be taken with administration of any new drug to a patient tak- ing FLU. Increased plasma levels of clozapine, lithium, and olanzapine have been observed with co-administration. In addition, FLU increases the halflife of diazepam. FLU has also shown to be an inhibitor of MAO enzymes, therefore, care should be taken when consid- ering co-administration with MAOIs.17
Citalopram
Citalopram is used to treat symptoms of major depression and it is also being evaluated in treatment of some anx- iety disorders. Concomitant use of antifungal medications and erythromycin can increase levels of citalopram. In addition, administration of citalopram with MAOIs can cause serotonin syndrome, characterized by at least three of the following symptoms: diarrhea, fever, sweating, mood or behavior changes, overactive reflexes, fast heart rate, restlessness, shivering or shaking.17
Paroxetine
Paroxetine is probably the most selective SSRI, relatively safe regarding cardiovascular effects, and is often used in elderly patients. About 95% of absorbed paroxetine is bound to plasma proteins, making interactions with other bound drugs such as clozapine and oral anticoagulants likely. Trials have shown an increase in bleeding after prolonged co-administration of paroxetine and warfarin.17
Patient History
A detailed history is the most effective way to prevent drug-drug interactions. Often, in the urgent care set- ting, it is difficult to obtain the entirety of a patient’s medical record. A thorough history can help prevent gaps in the medical record. Important factors to consider when reviewing the medication list include the patient’s age, the dosage of medications, and his/her usage of antibiotics. Although the urgent care provider in the patient scenario above appropriately prescribed an antibiotic other than penicillin because of the patient’s allergy, he did not recognize the interactions between warfarin and antibiotics. It is just as important to be aware of potential drug-drug interactions to avoid preventable complications. Table 2 suggests alternative antibiotics for common urgent care presentations in patients on warfarin therapy.
Age
Age is an important factor to consider in prevention of drug-drug interactions. Factors including frailty, memory loss, and use of many medications all contribute to the increased incidence of drug-related issues in older individuals.19 The prevalence of multiple chronic medical conditions requiring many different therapeutic protocols increases with old age, amplifying the chance of adverse drug-drug interactions.
Dosage
Adverse drug events, including dangerous drug-drug interactions, are commonly dose-related. To prevent dosage-related drug interactions, it is important as a provider to be aware of a number of factors that contribute to drug maintenance, including a patient’s nutritional status, degree of compliance, hepatic and renal function, and potential genetic factors contributing to the individual’s response to a particular drug.20
Conclusion
With the increasing number of drugs on the market and the prevalence of lengthy medication lists, it is unrealistic to expect an urgent care provider to remember all potential drug interactions. However, it is necessary to maintain a high level of suspicion when mak- ing changes or additions to a patient’s medications. Care must be taken to thoroughly review existing medications—both conventional and nonconventional— when prescribing a new medication. Extra precautions should be taken when treating elderly patients who may be on multiple drug therapies. ■
References
- FY 2012 Innovative Drug U.S. Food and Drug Administration. Web.
<http://www.fda.gov/downloads/aboutfda/reportsmanualsforms/reports/ucm330859.pdf>
- Ament P, Bertolino J, Liszewski Clinically significant drug interactions. Am Fam Physi- cian. 2000;61:1745-1754.
- Zhu J, Weingart, S. Prevention of adverse drug events in hospitals. Up to Date. http://www.uptodate.com/contents/prevention-of-adverse-drug-events-in- hospitals?source=search_result&search=risk+factors+for+adverse+drug+interactions&se lectedTitle=2~150
- Ji Y, Hokayem Moxifloxacin-warfarin interaction. J Comm Hosp Int Med Perspect. 2011.
- Becker Adverse drug reactions. Anesth Prog. 2011; 58: 31–41.
- Zithromax (azithromycin) 200 mg Tablet February U.S. Food and Drug Admin- istration. Web. http://www.fda.gov/Safety/MedWatch/SafetyInformation/Safety-Related- DrugLabelingChanges/ucm133116.htm
- De Smet P, Hofman A, Kasbergen A, Penning-van Bees F, Stricker B, Visser L, Vulto
- Overanticoagulation associated with combined use of antibacterial drugs and aceno- coumarol or phenprocoumon Thromb Haemost. 2002; 88: 705-710.
- Heiman H, Hylek E, Sheehan M, Singer D, Skates Acetaminophen and other risk factors for excessive warfarin anticoagulation. JAMA. 1998;279(9):657-662.
- Gallus A, Gebauer M, Henschke P, Nyfort-Hansen Warfarin and acetominophen interactions. Pharmacotherapy. 2003;23(1):109-112.
- Bixler F, Cheetham, T, Levy G, Niu Gastrointestinal safety of nonsteroidal antiin- flammatory drugs and selective cyclooxygenase-2 inhibitors in patients on warfarin. Ann Pharmacother. 2009;43:1765-1773.
- Valentine K, Hull Correcting excess anticoagulation after warfarin. Up to Date. Web http://www.uptodate.com/contents/correcting-excess-anticoagulation-after-warfarin
- Hansten P, Horn Drug interactions with antibacterial agents. J Fam Pract. 1995;41:81.
- Altman R, Dickinson B, Nielsen N, Sterling Drug interactions between oral con- traceptives and antibiotics. Obstet Gynecol. 2001;98:853-860.
- Understanding and using the S. Medical Eligibility Criteria for Contraceptive Use, 2010. Committee Opinion No. 505. American College of Obstetricians and Gynecolo- gists. Obstet Gynecol 2011;118:754–60.
- Perucca, Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Phar- macol. 2006;61(3):246–255.
- Bellosta S, Corsini Statin drug interactions and related adverse reactions. Expert Opin Drug Saf. 2012;11(6): 933-946.
- Mandriolo R, Mercolini L, Raggi M, Saracino Selective Serotonin Reuptake Inhibitors (SSRIs): Therapeutic Drug Monitoring and Pharmacological Interactions. Curr Med Chem. 2012;19:1846-1863.
- Clark Elevated international normalized ratio values associated with concomitant use of warfarin and ceftriaxone. Am J Health Syst Pharm Sep 1, 2011; 68(17):1603-1605.
- Huang A, Mallet L, Spinewine The challenge of managing drug interactions in eld- erly people. Lancet. 2007;370:185.
- Valentine K, Hull Therapeutic use of warfarin. Up to Date. Web. http://www.upto- date.com/contents/therapeutic-use-of-warfarin?source=search_result&search=War- farin+ plus+acetylsalicylic&selectedTitle=3~150#

