Yijung Russell, MD; Casey Collier, MD; Steve Christos, DO; and Shu B. Chan MD, MS
Citation: Russell Y, Collier C, Christos S, Chan SB. Active COVID-19 infection is indicated by WBC 7.0 and PLT
200 at presentation. J Urgent Care Med. 2021;15(10):35-38.
Introduction
The impact coronavirus disease of 2019 (COVID-19) has had on individuals, businesses, and governments is unprecedented in many ways. Though widespread and frequent screening is recommended for better containment,1 limited availability of testing supplies was a reality for many providers in emergency departments and urgent care centers, particularly in low-resource settings.
Cytopenia was associated with COVID-19 in early studies.2,3 As complete blood count (CBC) is almost always early in a visit and is also often the earliest test to result, it could be a valuable tool.
This retrospective review of ED visits had the objective of characterizing the association between different cell counts and active COVID-19 infection. Specifically, we examined white blood cell (WBC) and platelet (PLT) counts at presentation. We hypothesized that patients who presented with COVID-19 would be more likely to have relative leukopenia and thrombocytopenia.
Methods
Following IRB approval, data were abstracted by retrospective review of our electronic medical records from all ED visits at three community hospitals, all sites of a single emergency medicine residency program, from March 15, 2020 to April 15, 2020.
Included were all adult patients (age >18) presenting to the ED who had RT-PCR testing for SARS-CoV-2 (Abbott Molecular, Des Plaines, IL, USA) sent from the ED and subsequently admitted to the hospital. Excluded were those with history of liver or hematopoietic system disease and patients with missing data in the encounter. Abstracted data included demographics, chief complaints, ED vital signs, WBC count, PLT count, and result of COVID-19 testing.
With power set at 0.80 and significance set at 0.05, a sample size of 340 patients would detect a 15% difference among variables related to a positive SARS-CoV-2 test. Differences between positive and negative patients were tested using Student’s t-test and Chi-square test as appropriate. Significance was set at p=0.05 with Bonferroni correction when appropriate. Receiver operating characteristic (ROC) curve analysis was used to define optimum cutoff points for prediction of SARS-CoV-2 test results based on WBC and PLT counts. Sensitivity, specificity, positive, and negative likelihood ratios are reported with 95% confidence intervals. Selected variables were manually re-abstracted on 10% of random patients with a Kappa of 100%.
Results
Four hundred ninety-four patients met the inclusion criteria with 43.1% of cases from Site 1, 25.8% from Site 2, and 31.1% from Site 3. Patients with history of liver or hematopoietic system disease were excluded (n=13; cirrhosis (n=4), thrombocytopenia (n=6), multiple myeloma (n=2), lymphoma (n=1)). Five patients with encounters with missing data were also excluded, leaving a sample size of 476.
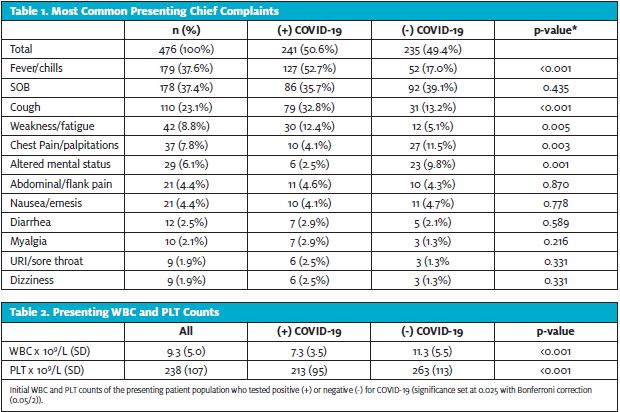
SARS-CoV-2 was detected in 241 patients (50.6%). The most common chief complaints were shortness of breath (29.6%), fever/chills (27.5%), and cough (10.7%). Diarrhea was not a common complaint and there were no reported symptoms of dysgeusia or anosmia (Table 1). Patients with COVID-19 were significantly younger (65.7 vs 70.4 years of age; p=0.002) and had higher incidence of fever/chills (p<0.001). Finally, COVID-19 patients had significantly lower presenting WBC (7.3 vs 11.3; p<0.001) and PLT counts (213 vs 263; p<0.001) (Table 2).
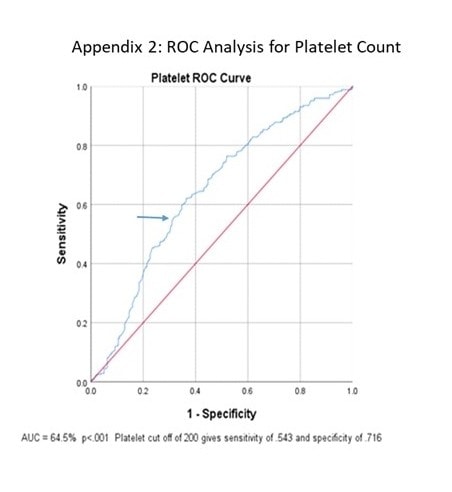
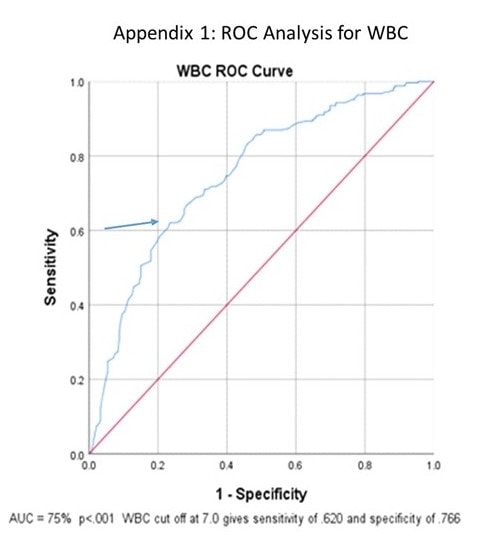
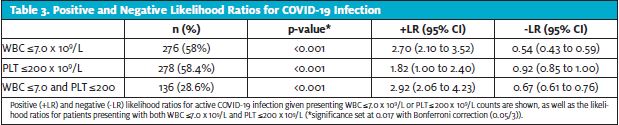
ROC curve analysis of initial WBC and PLT counts were both significant (p<0.001) with areas under the curve of 75% and 64.5% (Figure 1 and Figure 2). Further analysis showed that with a presenting WBC count of 7.0 x 109/Lor less, a patient was likely to be positive for COVID-19 with sensitivity of 0.620 and specificity of 0.766. In addition, with presenting WBC 7.0 x 109/L, the positive likelihood ratio of testing positive for COVID-19 was 2.70 (p<0.001, 95% CI: 2.10 to 3.52) and the negative likelihood ratio was 0.54 (p<0.001, 95% CI: 0.43 to 0.59) (Table 3).
Similarly, with a presenting platelet count of 200 x 109/L or less, a patient was likely to be positive for COVID-19 with sensitivity of 0.543 and specificity of 0.716, with a positive likelihood ratio of 1.82 (p<0.001, 95% CI: 1.00 to 2.40) and negative likelihood ratio of 0.92 (p<0.001, 95% CI: 0.85 to 1.00). When combined, WBC ≤7.0 x 109/L and platelet ≤200 x 109/L gave a sensitivity of 0.437 and specificity of 0.849 with positive likelihood ratio of 2.90 (p<0.001, 95% CI: 2.06 to 4.23) and negative likelihood ratio of 0.67 (p<0.001, 95%CI: 0.61 to 0.76).
Discussion
As clinicians, we triage and assign risks. We often have to make treatment and disposition decisions in real time, while waiting for a critical test, such as a “rapid” COVID-19 test. The strain of caring for critically ill patients can be augmented by limited resources as providers brace for additional surges of the pandemic. Any available data that could be utilized to decrease the cognitive burden of decision-making could be valuable as stress and burnout rose in response to COVID-19 [4,5].
Many clinicians and researchers have documented an association of leukopenia and thrombocytopenia with COVID-19 diagnosis and, often, an increased severity of illness.
Leukopenia is driven by lymphopenia, decrease of the lymphocytes. Angiotensin-converting enzyme expression in myeloid precursors causes macrophages to be more pro-inflammatory. This can then worsen immune response of target cells and cause consumption of T cells, especially CD4 and CD8 T cells.6 Thrombocytopenia in COVID-19 patients is thought to result from increased platelet consumption, low-grade disseminated intravascular coagulation, and in some cases a thrombotic microangiography. This is thought to be from endothelial damage releasing vWF multimers, activating platelets and increasing consumption.7 Others suggest the additional possibility of direct platelet stimulation by the SARS-CoV-2 spike protein.8
Our observation of significantly lower WBC and PLT counts in active COVID-19 infection is in agreement with previous studies.9-14 However, there have been very few studies giving the detailed association or, more important, the clinical implications. Our study suggests that the simple and rapidly available CBC, along with memorable cutoff points for presenting WBC and PLT counts, may provide the clinician with a useful tool for guiding clinical decision-making when test results are not easily available.
Limitations
There are several limitations to our study. The data reflect the conditions at the time of the study in three EDs in a single urban community and we cannot assert that our results would apply to a wider population.
In addition, we only examined patients who were symptomatic enough to be admitted, so it is unclear whether or not discharged patients with COVID-19 or those who did not require admission would have the same characteristics. The PCR test used to detect SARS-CoV-2 RNA (Abbott Molecular, Des Plaines, IL, USA) received Emergency Use Authorization (EUA) for a PCR test to detect SARS-CoV-2 RNA in nasopharyngeal and oropharyngeal swabs from patients with suspected COVID-19. Due to the EUA, manufacturer sensitivity and specificity is not provided. However, independent testing showed sensitivity at 93% and specificity at 100%.15
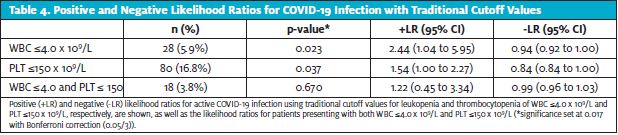
Finally, the traditional laboratory cutoff values that define leukopenia and thrombocytopenia are WBC 4.0 x 109/L and PLT
150 x 109/L, respectively. Using those cutoff values gave likelihood ratios that trended in the same direction as our main results (Table 4). However, the sample sizes with traditional cutoff values were much smaller and resulted in less robust data, so we decided to primarily present results with cutoff values that were nontraditional but simple to remember, with stronger evidence.
We would like to emphasize that our study supports association between active COVID-19 infection and presenting WBC and PLT counts that trend closer to the lower end of normal rather than actual leukopenia and thrombocytopenia as defined by traditional laboratory cutoffs. It is also important to note that the likelihood ratio should be used in context of the pretest probability specific to the patient, which will vary widely depending on presenting symptoms and local prevalence.
CONCLUSIONS
In this multicenter community study of ED patients admitted over 1 month with suspected COVID-19, both the initial WBC and PLT counts were significantly lower in patients with active COVID-19 infection. Our study shows that a symptomatic patient with presenting WBC and PLT counts ≤7.0 and 200, respectively, is almost three times more likely to be positive for active COVID-19 infection.
REFERENCES
- Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 test sensitivity—a strategy for containment. N Engl J Med. 2020;383(22):e120.
- Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36.
- Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145-148.
- Kannampallil TG, Goss CW, Evanoff BA, et al. Exposure to COVID-19 patients increases physician trainee stress and burnout. PLoS One. 2020;15(8):e0237301.
- Barello S, Palamenghi L, Graffigna G. Burnout and somatic symptoms among frontline healthcare professionals at the peak of the Italian COVID-19 pandemic. Psychiatry Res. 2020;290:113129.
- Liu X, Zhang R, He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann Hematol. 2020;99(7):1421-1428.
- Amgalan A, Othman M. Exploring possible mechanisms for COVID-19 induced thrombocytopenia: unanswered questions. J Thromb Haemost. 2020 Jun;18((6)):1514–6.
- Zhang S, Liu Y, Wang X, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13(1):120.
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19). Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623.
- da Silva SJR, Silva CTAD, Guarines KM, et al. Clinical and laboratory diagnosis of SARS-CoV-2, the virus causing COVID-19. ACS Infect Dis. 2020;6(9):2319-2336.
- Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS‐CoV‐2 infection: review of 3939 COVID‐19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020; 108:17-41.
- Warkentin TE, Kaatz S. COVID-19 versus HIT hypercoagulability. Thrombosis Research. 2020;196:38-51.
- Bomhof G, Mutsaers PGNJ, Leebeek FWG, et al. COVID‐19‐associated immune thrombocytopenia. Br J Haematol. 2020;190:e61-e64.
- Wool GD, Miller JL. The Impact of COVID-19 disease on platelets and coagulation pathobiology. 2020;88(1):15-27.
- Degli-Angeli E, Dragavon J, Huang ML, et al. Validation and verification of the Abbott RealTime SARS-CoV-2 assay analytical and clinical performance. J Clin Virol. 2020;129:104474.
Author affiliations: Yijung Russell, MD; Casey Collier, MD; Steve Christos, DO; and Shu B. Chan MD, MS are affiliated with AMITA Presence Resurrection Medical Center Chicago. Dr. Russell is partnered with the editor-in-chief of JUCM; all procedures have been followed to ensure blinded review. The authors have no relevant financial relationships with any commercial interests.
Read More on COVID-19
- Incidence Of SARS-CoV-2 In Preoperative Patients Tested In An Urgent Care Setting
- Breakthrough COVID Infection In A Vaccinated Person From A Possible Vaccinated Exposure
- Outpatient Management Of COVID-19 In The Urgent Care Clinic: Administering Monoclonal Antibodies