Published on
Download the article PDF: A Rare Cardiopulmonary Debut Of Systemic Lupus Erythematosus A Pediatric Case Report
Urgent Message: In adolescents presenting with persistent chest pain and systemic symptoms, clinicians should maintain a high index of suspicion for autoimmune etiologies such as systemic lupus erythematosus causing pericardial and pleural effusions.
Asra Usmani, MBBS, MD; Ali Baidoun, MD; Aaron Mahoney, DO; Megan Sikkema, DO
Key Words: Pericardial Effusion, Pleural Effusion, Systemic Lupus Erythematosus, Chest Pain, Adolescent
Abstract
Introduction: Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that can affect multiple organ systems. Its clinical presentation ranges from mild with localized symptoms to life-threatening, multiorgan system involvement. Early recognition is essential to initiate prompt treatment and prevent disease progression.
Clinical Presentation: A 16-year-old, previously healthy girl presented urgently to her pediatrician with a 4-week history of persistent left-sided chest pain. She was initially treated for presumed gastroesophageal reflux disease with omeprazole after presenting on December 5, 2023. Over the following 4 weeks she developed intermittent fevers, shortness of breath, and pleuritic chest pain. A chest x-ray on December 29, 2023, revealed bilateral pleural effusions and left lower lobe consolidation. Despite antibiotics, symptoms worsened, leading to admission on January 2, 2024.
Diagnosis: Upon worsening symptoms, she was admitted, and computed tomography imaging revealed a large pericardial effusion and a significant left pleural effusion causing near-complete lung collapse. She was transferred to the pediatric intensive care unit and underwent urgent pericardiocentesis with pericardial drain placement and left chest tube drainage. Subsequent autoimmune workup revealed a high antinuclear antibody titer (>1:640, speckled pattern), elevated anti-double stranded DNA antibodies, and inflammatory markers consistent with a new diagnosis of SLE with serositis.
Resolution: The patient was initiated on high-dose corticosteroids, which resulted in rapid clinical improvement. The pericardial and pleural effusions resolved, and the patient was discharged with a tapering steroid regimen and started on hydroxychloroquine and azathioprine with rheumatology follow-up.
Conclusion: Concurrent pericardial and pleural effusions as the initial presentation of pediatric SLE is rare. This case underscores the importance of maintaining a broad differential diagnosis in adolescents presenting with persistent chest pain and systemic symptoms. Early use of imaging and autoimmune serologies can facilitate timely diagnosis and initiation of appropriate immunosuppressive therapy.
Introduction
Systemic lupus erythematosus (SLE) is a complex, chronic autoimmune disease characterized by the production of autoantibodies and multiorgan involvement. It can present with a wide array of clinical manifestations, ranging from subtle constitutional symptoms to fulminant organ failure. Although the disease predominantly affects women of reproductive age, approximately 15–20% of cases present during childhood or adolescence, where the disease course tends to be more severe and systemic in nature.1,2
Cardiopulmonary involvement is common in SLE but is often under-recognized during initial evaluations, especially in pediatric patients presenting with nonspecific symptoms such as fatigue, fever, and chest pain.3 Pericarditis is the most frequent cardiac manifestation of lupus and may occur in up to 25% of pediatric SLE patients during the disease course.4 Pleural effusions are also frequently observed in SLE but are rarely the initial presenting feature.5 The simultaneous occurrence of both pericardial and pleural effusions as an initial presentation of lupus, especially without a previously established diagnosis, is exceedingly rare and can obscure the underlying etiology.6
Early recognition and diagnosis of SLE are crucial to prevent potentially life-threatening complications and organ damage.7 When presenting symptoms mimic more common conditions, such as viral infections, pneumonia, or gastroesophageal reflux disease, it is imperative for clinicians, especially those in urgent care and primary care settings, to maintain a broad differential diagnosis. Inflammatory markers, serological testing, and timely imaging can help unveil atypical presentations and guide appropriate referrals.
This case report discusses a 16-year-old girl who presented with both pericardial and pleural effusions, leading to the diagnosis of SLE. Similar cases in the literature reinforce the importance of recognizing serosal effusions as early signs of SLE. Yost et al. described a patient with known SLE who developed recurrent pleural and pericardial effusions requiring repeated drainage and immunosuppression.8 In another report, a patient with undiagnosed SLE and COVID-19 presented with similar findings, complicating the diagnosis.9 Misra et al. reported pediatric cases where pericarditis and serositis were the first signs of SLE, often misdiagnosed initially.10 These cases highlight the diagnostic value of early recognition of sterile, exudative effusions in prompting autoimmune evaluation, particularly in pediatric populations. This case underscores the importance of considering autoimmune etiologies in patients with unexplained serosal effusions and highlights the need for a high index of suspicion to facilitate timely diagnosis and management.
Case Presentation
A 16-year-old, previously healthy girl presented urgently to her pediatrician due to worsening left-sided chest pain that had been ongoing for approximately 4 weeks. The patient was noted to be of mixed ethnicity, and family history was notable for type 1 diabetes mellitus in the maternal grandmother and older sister, who also had celiac disease. There was no known family history of SLE, rheumatoid arthritis, psoriasis, or inflammatory bowel disease. At that initial visit, she was diagnosed with gastroesophageal reflux disease (GERD) and managed conservatively with omeprazole 20 mg daily.
Despite this, her symptoms persisted and gradually worsened. Over the following 2 weeks, she developed intermittent fevers with a peak temperature of 102.6°F (39.2°C), pleuritic chest pain radiating to the back, shortness of breath, and left breast discomfort. She was re-evaluated for a presumed viral upper respiratory tract infection; however, viral panels were negative, and no infectious etiology was found. Given the persistence and evolution of her symptoms, a chest x-ray was obtained. Imaging revealed left lower lobe consolidation suggestive of pneumonia, along with a moderate left pleural effusion and a small right pleural effusion (Figure 1). She was started on a course of amoxicillin and inhaled albuterol.
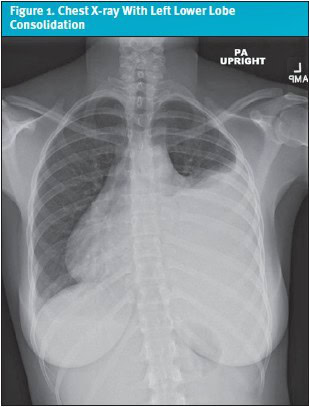
Despite initiation and compliance to treatment, her symptoms did not improve, and her chest pain further intensified significantly. This prompted patient to present for the urgent visit, at which time, given concern for worsening pulmonary pathology, she was urgently admitted for further workup.
Upon hospital admission, vitals were: blood pressure of 113/74; heart rate of 121 beats per minute; respiratory rate of 22 breaths per minute; temperature of 99.7°F (37.6°C), O2 saturation of 98% on room air. She was ill-appearing, tachycardic, with diminished breath sounds over the left lung fields. A bedside point-of-care chest ultrasound revealed a large left pleural effusion with complex echogenicity concerning for an underlying pericardial effusion or necrotizing pneumonia. A computed tomography (CT) chest scan with contrast was promptly obtained, which demonstrated a large pericardial effusion, a significant left-sided pleural effusion, and near-complete left lung collapse (Figure 2). Given the severity of these findings, the patient was transferred to the pediatric intensive care unit (PICU) for advanced care.
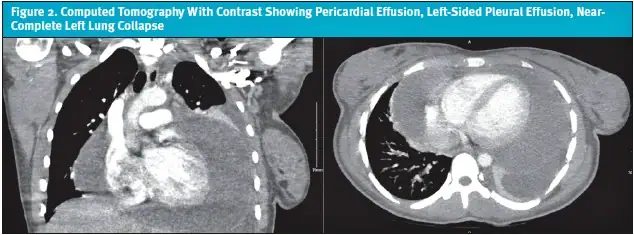
In the PICU, a bedside echocardiogram confirmed a large pericardial effusion without signs of tamponade. Cardiology was consulted, and the patient was urgently taken to the cardiac catheterization lab for therapeutic and diagnostic pericardiocentesis, during which 800 mL of serosanguinous fluid was removed. A pericardial drain was left in place for continued monitoring. Simultaneously, a left-sided chest tube was placed due to moderate dyspnea with minimal movement, and approximately 550 mL of pleural fluid was drained. Initial laboratory evaluation revealed a microcytic anemia, elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), and a neutrophilic predominance in pericardial fluid, without any identified organisms on Gram stain or culture. An autoimmune workup was initiated due to the exudative and inflammatory nature of the pericardial fluid. Notably, her antinuclear antibody (ANA) returned strongly positive at 1:640 (speckled pattern) with anti-Sjögren-syndrome-related antigen A (anti-SSA) positivity and low complement component 4 (C4) levels. Additional autoimmune markers were sent.
Given the serological findings and the clinical picture of polyserositis (pericarditis and pleuritis), the patient was diagnosed with new-onset SLE. She was started on high-dose corticosteroids (methylprednisolone 30 mg/kg/day for 3 days) and transitioned to scheduled non-steroidal anti-inflammatory drugs (NSAIDs) for pain control. She showed rapid clinical improvement. The pericardial drain was removed on day 2, and the chest tube on day 3, following resolution of effusions confirmed on repeat echocardiogram and chest imaging (Figure 3).
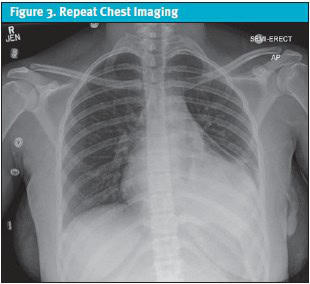
The patient was discharged on a tapering dose of steroids with outpatient follow-up by pediatric rheumatology. At follow-up with rheumatology, it was assessed that her SLE was clinically active on medications as evidenced by ongoing symptoms. She was started on hydroxychloroquine and azathioprine with a steroid taper. No ECG was obtained at outpatient visits or during hospital admission, where management focused on urgent drainage and stabilization. The patient was monitored on continuous telemetry in the PICU, though tracings were not preserved. The first formal ECG was performed approximately 4 weeks after symptom onset, by which time she had improved clinically. It demonstrated normal sinus rhythm with respiratory sinus arrhythmia and mild nonspecific T-wave abnormalities (Figure 4). Notably, it did not show findings typically associated with acute pericarditis or large pericardial effusion, such as diffuse ST-segment elevation or PR depression. Patient was managed on hydroxychloroquine and azathioprine until later follow-ups with rheumatology revealed that her SLE was clinically quiescent with a SELDAI-2K (Systemic Lupus Erythematosus Disease Activity Index) score of 0, normalized complements, and no recurrence of pericardial or pleural effusions.
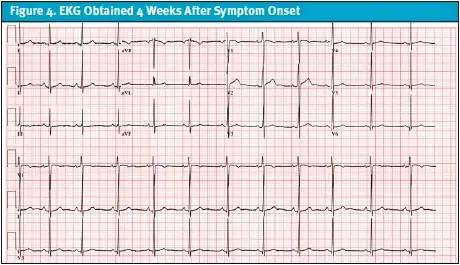
In summary, the patient’s illness evolved over the course of 4 weeks. She was first seen on December 5, 2023, in her pediatrician’s office with chest pain and diagnosed with GERD, for which she was started on omeprazole. Ten days later, on December 15, she returned with fever and upper respiratory symptoms. Viral testing was negative, and she was managed conservatively. On December 29, a third pediatrician office visit prompted chest radiography, which demonstrated pneumonia with bilateral pleural effusions, and she was prescribed amoxicillin and albuterol. Despite treatment, her symptoms worsened, and on January 2, 2024, she presented to urgent care with escalating chest pain, dyspnea, and fever. This ultimately led to her admission, advanced imaging, identification of large pericardial and pleural effusions, and transfer to the PICU for urgent drainage and further evaluation.
Medical Decision Making
In the outpatient setting, medical decision making initially focused on common diagnoses such as GERD (visit 1) and viral or bacterial respiratory infection (visits 2 and 3). These represent common and reasonable considerations in adolescents with chest pain. However, the persistence of symptoms despite therapy, evolution to pleuritic pain, and association with fever were red flags suggesting a broader differential. For urgent care and primary clinicians, differentials for adolescent chest pain with systemic features should include pneumonia with effusion, viral pericarditis, myocarditis, autoimmune serositis, and less commonly, oncologic processes.
In the hospital, the patient’s workup rapidly evolved due to the unexpected severity and chronicity of her chest symptoms. The differential included infectious causes (bacterial, viral, atypical organisms), inflammatory, and autoimmune etiologies. Imaging played a pivotal role in detecting pericardial and pleural fluid collections. Given her hemodynamic stability but large pericardial effusion, pericardiocentesis was pursued to prevent tamponade and clarify etiology.
With no organisms seen on Gram stain and no growth in cultures, and pericardial fluid characteristics suggestive of an exudative inflammatory process, autoimmune causes became a priority. Strong ANA positivity with specific extractable nuclear antigens (SSA) and low complement supported a diagnosis of SLE with serositis. Collaborative management between cardiology, infectious diseases, and rheumatology enabled timely initiation of high-dose steroids, followed by immunomodulatory therapy with azathioprine and hydroxychloroquine.
Differential Diagnosis and Final Diagnosis
At hospital admission, differential diagnoses considered included:
- Infectious pericarditis (bacterial, viral, atypical pneumonia-related)
- Parapneumonic effusion/empyema
- Reactive pericarditis post-viral illness
- Autoimmune serositis (lupus pericarditis)
- Oncologic processes (lymphoma, leukemia)
The final diagnosis was determined to be SLE presenting with pericarditis and pleuritis, confirmed by:
- Positive ANA (1:640, speckled pattern)
- SSA, anti-dsDNA, chromatin antibodies
- Hypocomplementemia
- Clinical criteria of serositis (pericardial and pleural effusion)
Discussion
This case highlights the diagnostic complexity and urgency surrounding a seemingly common chief complaint, chest pain, in an adolescent female. While musculoskeletal and gastrointestinal causes often predominate in this age group, the evolution of symptoms over a 4-week period, including pleuritic pain, dyspnea, and intermittent fevers, demanded deeper investigation.
Epidemiologically, SLE is a rare but important cause of pericarditis and pleuritis in pediatric patients, with peak incidence during adolescence and a strong female predominance.6 Serositis is a well-described manifestation of SLE and may present as pericardial or pleural effusions, sometimes preceding the formal diagnosis of lupus.11 In this case, SLE initially presenting as isolated chest pain mimicked benign or infectious etiologies, including GERD, pneumonia, and viral pericarditis, which lead to initial misdiagnoses and outpatient treatment with antibiotics and proton pump inhibitors.
Key to this patient’s diagnostic turnaround was her return to care due to worsening symptoms. Point-of-care ultrasound and chest imaging upon urgent admission to the hospital revealed bilateral pleural effusions and a large pericardial effusion, prompting escalation of care and transfer to the PICU. The pericardial effusion was large enough to risk progression to tamponade, justifying urgent pericardiocentesis, which was both therapeutic and diagnostic. Fluid analysis revealed a sterile, neutrophil-predominant exudate, and the presence of systemic inflammation prompted autoimmune serologies. The subsequent ANA titer (1:640) and SSA positivity, with low complement levels, confirmed the diagnosis of new-onset pediatric SLE presenting with serositis.
Although ECG is a key diagnostic tool in suspected pericarditis or pericardial effusion, its sensitivity depends on the timing of acquisition relative to symptom onset. In this case, the ECG was obtained 4 weeks after the initial presentation and did not demonstrate classical findings of pericarditis. Earlier ECGs in similar cases may reveal low-voltage QRS complexes or electrical alternans in the setting of large effusions. This case underscores the importance of timely ECG evaluation in adolescents presenting with unexplained chest pain, even when vital signs and exam findings are initially nonspecific.
Published cases suggest that early recognition of sterile, exudative pleural or pericardial effusions, particularly when unresponsive to antibiotics, should prompt autoimmune workup. Yost et al. noted that recurrent serosal effusions in known SLE can indicate disease activity, while Misra et al. described pediatric patients whose first SLE sign was pericarditis or polyserositis. Similarly, Amro et al. reported diagnostic delay in a case of lupus complicated by COVID-19.8-10 In our case, the combination of unexplained polyserositis, elevated inflammatory markers, and negative infectious workup paralleled these patterns and helped guide rheumatologic evaluation and diagnosis.
This case underscores the value of revisiting the differential diagnosis when symptoms persist beyond the expected course, especially in adolescents presenting repeatedly with chest pain and systemic symptoms. Urgent care clinicians are often the first point of contact for such patients and play a pivotal role in recognizing red flags (eg, pleuritic quality, systemic features, poor response to treatment) that should prompt escalation to imaging and specialist referral. Correct diagnosis and multidisciplinary care coordination led to management of this potentially life-threatening autoimmune condition.
Disposition
The patient was discharged home in stable condition on hospital day 4 after pericardial and pleural fluid drainage with no recurrence on follow-up echocardiograms. She was started on high-dose corticosteroids, hydroxychloroquine, and azathioprine with a tapering steroid plan. At 2-week rheumatology follow-up, her SLE was assessed as active with a SELDAI-2K score of 17. She was managed and followed by rheumatology while remaining on azathioprine and hydroxychloroquine. A 2-month follow-up revealed that her SLE was clinically quiescent with a SELDAI-2K score of 0, normalization of complements and anti-dsDNA, and no recurrence of pericardial or pleural effusions. She remains on close follow-up with rheumatology for continued immunosuppressive therapy and disease monitoring.
Ethics Statement
Informed written consent was obtained from the parent of the patient for publication of this case.
Takeaway Points
- Persistent chest pain with systemic features in adolescents warrants reassessment—especially when initial treatment fails.
- Lupus pericarditis can present as isolated chest pain with pleural effusion and may mimic pneumonia or viral illness early in its course.
- Point-of-care ultrasound, chest imaging, and early electrocardiography are critical tools in urgent care clinics to detect pericardial and pleural effusions that may require emergent intervention.
- A large pericardial effusion in a stable patient still requires prompt evaluation due to the risk of tamponade, even in the absence of classic signs.
- Early rheumatologic evaluation is essential when pericardial effusion is exudative and sterile, especially with positive ANA or hypocomplementemia.
Manuscript submitted April 22, 2025; accepted September 11, 2025.
References
- Nusbaum JS, Mirza I, Shum J, et al. Sex differences in systemic lupus erythematosus: epidemiology, clinical considerations, and disease pathogenesis. Mayo Clin Proc. 2020;95(2):384-394. doi:10.1016/j.mayocp.2019.09.012
- Aggarwal A, Srivastava P. Childhood onset systemic lupus erythematosus: how is it different from adult SLE? Int J Rheum Dis. 2015;18(2):182-191. doi:10.1111/1756-185X.12419
- Barradas MI, Duarte F, dos Santos IC, et al. Cardiac Tamponade as the First Manifestation of a Systemic Disease. Int J Clin Cardiol. 2023;10:287. doi:10.23937/2378-2951/1410287
- Maharaj SS, Chang SM. Cardiac tamponade as the initial presentation of systemic lupus erythematosus: a case report and review of the literature. Pediatr Rheumatol Online J. 2015;13:9. doi:10.1186/s12969-015-0005-0
- De Matteis A, Sacco E, Celani C, et al. Tocilizumab for massive refractory pleural effusion in an adolescent with systemic lupus erythematosus. Pediatr Rheumatol Online J. 2021;19(1):144. doi:10.1186/s12969-021-00635-w
- Chen YJ, Lin YJ. Pediatric Lupus Presenting as Pulmonary Hypertension, Myocarditis, and Massive Pericardial Effusion in an 11-Year-Old Girl: A Case Report and Literature Review. Front Pediatr. 2022;10:829166. doi:10.3389/fped.2022.772422
- Mitchell JL. Understanding the impact of delayed diagnosis and misdiagnosis of systemic lupus erythematosus (SLE). J Family Med Prim Care. 2024;13(11):4819-4823. doi:10.4103/jfmpc.jfmpc_1177_24
- Yost C, Vercillo D, Abuqare A, Yost MB, Love AN, Vercillo DM. Pleural and Pericardial Effusion With COVID-19 and Systemic Lupus Erythematosus and Its Recurrence: A Case Study. Cureus. 2023 Apr 22;15(4). doi: 10.7759/cureus.37988
- Amro AM, Deeb S, Rije R, Deeb N, Qunaibi YY, Amr B, Irzeqat K, Alhadad B, Emar A, Deeb S. Systemic lupus erythematosus presenting as cardiac tamponade and pleural effusion: a case report. Cureus. 2024 Jan 25;16(1). doi:10.7759/cureus.52894
- Ryu S, Fu W, Petri MA. Associates and predictors of pleurisy or pericarditis in SLE. Lupus Science & Medicine. 2017 Oct 1;4(1):e000221. doi:10.1136/lupus-2017-000221
- Harrison MJ, Zühlke LJ, Lewandowski LB, Scott C. Pediatric systemic lupus erythematosus patients in South Africa have high prevalence and severity of cardiac and vascular manifestations. Pediatr Rheumatol. 2019;17(1):1-10. doi:10.
Author Affiliations: Asra Usmani, MBBS, MD, Department of Pediatrics, Western Michigan University Homer Stryker, MD, School of Medicine, Kalamazoo, Michigan. Ali Baidoun, MD, Department of Pediatrics, Western Michigan University Homer Stryker, MD, School of Medicine, Kalamazoo, Michigan. Aaron Mahoney, DO, Department of Emergency Medicine, Western Michigan University Homer Stryker, MD, School of Medicine, Kalamazoo, Michigan. Megan Sikkema, DO, Department of Pediatrics, Western Michigan University Homer Stryker, MD, School of Medicine, Kalamazoo, Michigan, and Bronson Children Hospital, Kalamazoo, Michigan. Authors have no relevant financial relationships with any ineligible companies.
Read More
