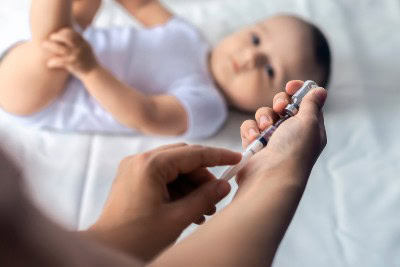
In a study of 31,900 healthy term infants at Kaiser Permanente Northern California during the 2023–2024 respiratory syncytial virus (RSV)-season, researchers found nirsevimab demonstrated high effectiveness in preventing RSV-associated lower respiratory tract disease (LRTD), as published in Pediatrics. Among immunized infants (49.1%), the incidence of RSV-associated LRTD was 6.10 per 1,000 person-years compared to 58.51 in non-immunized infants (95% confidence interval [CI], 81.7%–91.1%; P<0.001). Effectiveness against hospitalized RSV LRTD was 98.0% (95% CI, 85.1%–99.7%). Immunized …
Read More