Published on
Download the article PDF: Shortness Of Breath In Pregnancy Differentiating Physiology From Pathology In Urgent Care
Urgent Message: As shortness of breath and edema are common in pregnancy, urgent care clinicians must distinguish patients with normal physiologic changes from those with emergent conditions like venous thromboembolism, hypertension, eclampsia, and cardiomyopathy.
John Ramos, MMS, PA-C, CAQ-EM
Key Words: Ambulatory Care; Differential Diagnosis; Dyspnea; Pregnancy Complications; Pregnancy Complications, Cardiovascular
Abstract
Background: Physiologic changes in pregnancy contribute to shortness of breath and edema. However, these symptoms can also be caused by serious etiologies including venous thromboembolism (VTE) (increased risk in pregnancy and a leading cause of maternal mortality), preeclampsia and eclampsia, cardiomyopathy, and valvular heart disease.
Aim: This review aims to increase urgent care clinician awareness of the differential diagnoses associated with shortness of breath or edema in pregnancy, with special consideration for the diagnosis and management of VTE.
Conclusion: It is essential to consider thromboembolic, hypertensive, and cardiac disorders when evaluating pregnant patients with shortness of breath or edema. Urgent care clinicians must be aware of diagnostic tests and treatment options for VTE as well as indications for emergency department referral.
Introduction
An estimated 60-70% of women experience shortness of breath (SOB) during pregnancy, although it typically does not interfere with daily activities or exercise tolerance.1 While SOB is commonly ascribed to physiologic changes in pregnancy (Table 1),2-4 clinicians must exercise caution to distinguish serious or life-threatening pathology. In the urgent care setting, history, physical examination, and diagnostic testing are used to differentiate benign from serious conditions to determine those who require emergency department (ED) evaluation. SOB may also occur because of traumatic injury (eg, rib fractures, pulmonary contusion, diaphragm rupture), which is typically associated with pain and is outside the scope of this article.

Differential Diagnoses for Shortness of Breath
Anemia
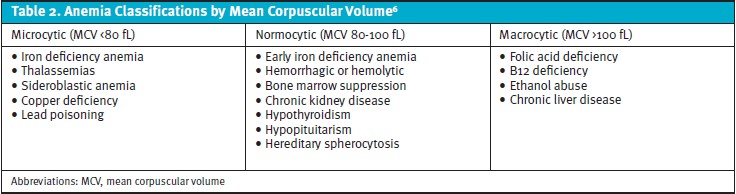
About 40% of pregnancies are complicated by some form of anemia.5 Anemia may be inherited (eg, hemoglobinopathy, thalassemia) or acquired (eg, nutrient deficiency, aplastic anemia). Anemia is also classified by mean corpuscular volume (Table 2). In addition to SOB, patients with anemia may also notice fatigue, lightheadedness, palpitations, and brittle nails. The diagnosis of an underlying hematologic disorder can be challenging in pregnancy as normal plasma volume expands by 40-50% and erythrocyte mass increases 15-25%.6 Anemia is diagnosed by a hemoglobin less than 11 g/dL in the first and third trimesters, and less than 10.5 g/dL in the second trimester. Screening for anemia should occur during the first trimester and at 24 weeks to 28 weeks of gestation.5 Nutritional deficiencies including iron and B12 should also be considered in pregnant women, especially those with a history of bariatric surgery.5
Ferritin <30 ng/L is the most sensitive and specific test for diagnosing iron deficiency anemia.6 The prevalence of anemia is highest in non-Hispanic Black mothers as well as teenage mothers of any race. The risk of low birth weight, preterm delivery, and perinatal mortality is increased among pregnant women with iron deficiency anemia.5,6
Asthma
Asthma is the most common chronic disease affecting pregnant women and complicates up to 8% of pregnancies.7 It is estimated that about one-fourth of pregnant women with chronic asthma will experience deterioration in asthma control due to physiologic changes of pregnancy including decreased tidal volume due to increased diaphragm elevation, increased oxygen demand (due to relative plasma expansion), and increased minute ventilation.8 Physiologic changes may declare a diagnosis of asthma in women previously undiagnosed.7,8 Exacerbation triggers include increased susceptibility to viral infections as well as hormone mediated rhinosinusitis and laryngeal edema (prevalent in up to 20% of pregnancies).9 Gastroesophageal reflux can also be a trigger, and symptoms of dyspepsia may appear or worsen in pregnancy due to progesterone mediated relaxation of the lower esophageal sphincter.8 Poorly controlled asthma in pregnancy has observed associations with low birth weight and intra-uterine growth restriction, as well as an increased risk of preeclampsia, gestational diabetes, preterm delivery, and caesarean delivery. Spirometry is used to diagnose and monitor asthma and typically shows evidence of a reversible obstructive pattern. Hospitalization is indicated for patients with forced expiratory volume in the first second (FEV1) less than 50% of predicted FEV1, altered sensorium, or hypercarbia (partial pressure of carbon dioxide >34 to 42 mm Hg). 8,10
Respiratory Infections
Infectious respiratory conditions should also be considered for pregnant women with SOB and/or fever, cough, rhinorrhea, or sore throat. These include viral/bacterial pneumonia and viral infections including influenza. In addition to fever, sputum production and pleuritic chest pain may be present in bacterial pneumonia. Physical exam may reveal crackles or wheezing.11 A chest x-ray may be considered to confirm the diagnosis of community acquired pneumonia or complications like parapneumonic effusion or empyema. Pathogen-specific viral testing may be seasonally indicated (eg, influenza, COVID-19), and empiric treatment of influenza is recommended if testing is not immediately available. Postexposure chemoprophylaxis for influenza may also be offered for patients with a history of close contact with infectious individuals.12
Obesity and Obstructive Sleep Apnea
Obesity is the most common medical condition impacting women of reproductive age.13 Obesity is defined as body mass index (prepregnancy weight in kilograms/height in meters) greater than or equal to 30.14 When prepregnancy weight is unknown, the initial prenatal visit weight is used to calculate body mass index.13 A gestational weight gain of 5.0-9.1 kilograms (11-20 pounds) is acceptable for women with obesity, and 6.8-11.3 kilograms (15-25 pounds) for those with body mass index of 25-29.9.13 Physiologically, increased adipose tissue leads to reduced lung volumes and increased respiratory effort. Obesity in pregnancy is associated with an increased risk of miscarriage and recurrent miscarriage, cardiac dysfunction, proteinuria, gestational diabetes, preeclampsia, nonalcoholic fatty liver disease, venous thromboembolism, and obstructive sleep apnea (OSA).13 Symptoms of OSA include snoring, excessive daytime sleepiness, and witnessed apneic episodes. OSA should be considered for pregnant women with unexplained hypoxia. When OSA is suspected, patients should be referred to a sleep medicine specialist for evaluation and management.13
Cardiovascular Conditions
A not insignificant number of pregnancies are complicated by cardiovascular disorders.15 Systemic vascular resistance (SVR) normally decreases in the first trimester reaching a nadir of 30% from baseline in the second trimester before returning to prepregnancy levels in the third trimester.16 Decreased SVR (afterload) and increased blood volume (preload) increase cardiac output by 50% in pregnancy to meet fetal and maternal metabolic needs.16 While edema and SOB can be normal pregnancy symptoms, abrupt changes in peripheral edema, weight gain, or decreased exercise tolerance may herald emergent pathology including preeclampsia, valvular heart disease, and heart failure.15,16 The presence of pulmonary edema (either auscultated crackles or radiographically identified), hypoxia, or increased respiratory effort warrant ED evaluation.
Hypertension
Hypertensive disorders complicate 2-8% of pregnancies and contribute to more than 50,000 maternal and 500,000 fetal deaths globally.15 Increased SVR associated with hypertension leads to increased capillary permeability, volume retention, and pulmonary edema. Of note, the diagnosis of gestational hypertension (HTN) may be obscured until the third trimester when SVR returns to prepregnancy levels.16 Gestational hypertension (HTN) may be first diagnosed at 20 weeks of gestation, and is defined as a systolic blood pressure (SBP) greater than or equal to 140 mm Hg or diastolic blood pressure (DBP) greater than or equal to 90 mm Hg on at least 2 occasions at least 4 hours apart.16 Severe gestational HTN is diagnosed by SBP greater than or equal to 160 mm Hg or DBP greater than or equal to 110 mm on at least 2 occasions at least 15 minutes apart.16 The presence of HTN and elevated protein (dipstick ≥2+, 24-hour urine ≥300 mg, urine protein to creatinine ratio ≥0.3) is diagnostic of preeclampsia. Eclampsia is recognized as gestational HTN in the presence of thrombocytopenia (platelets less than 100 x 109/L), hemolysis, elevated serum creatinine (greater than 1.1 mg/dl or doubling of baseline), transaminitis, pulmonary edema, or unexplained headache and/or visual symptoms.17
Cardiomyopathy
Pregnancy-associated cardiomyopathy (peripartum or postpartum cardiomyopathy) is a rare disorder characterized by new onset unexplained left ventricular (LV) systolic dysfunction (ejection fraction <45% with or without LV dilation on echocardiography) toward the end of pregnancy or in the postpartum period.18 The incidence of pregnancy-associated cardiomyopathy in the United States is about 1 case per 4,000 live births. While the diagnosis can be made at early gestational ages and up to 5 months postpartum, the majority of cases are diagnosed in the final month of pregnancy (19%) or the first month postpartum (75%). Forty-five percent of diagnoses occur in the first week after delivery.18 Pregnancy associated cardiomyopathy is more likely in women older than 30 years old, parity greater than or equal to 4, and multiple gestation pregnancies.19 Symptoms include SOB, decreased exercise tolerance, fatigue, orthopnea, pedal edema, and hemoptysis. Peripheral edema and jugular venous distention may be present on physical exam, and hypoxia and/or crackles are more likely with severe disease progression. In 1 large registry, pulmonary rales were present in 59% of patients, and 46% of patients had a third heart sound.20 Echocardiography is the preferred imaging modality for diagnosis. Brain natriuretic peptide is a useful initial laboratory test for clinically stable patients not warranting ED evaluation, although it may be falsely low in patients with obesity.19
Valvular Heart Disease
Valvular heart disease (VHD), with preserved or reduced ejection fraction, may also contribute to SOB or decreased exercise tolerance. Valvular heart disease is present in less than 1% of pregnancies. Stenotic lesions are not as tolerable as regurgitant lesions, owing to a 50% increase in the transvalvular gradient from increased cardiac output.20 Both regurgitant and stenotic lesions are associated with maternal and fetal complications. Structural intervention prior to pregnancy is recommended for women with at least moderate mitral stenosis. Emergent structural interventions may be required for women with symptoms of VHD refractory to medical therapy, hemodynamic instability, or acute onset of disease (eg, acute chordal rupture and severe mitral regurgitation).18,19
Venous Thromboembolism
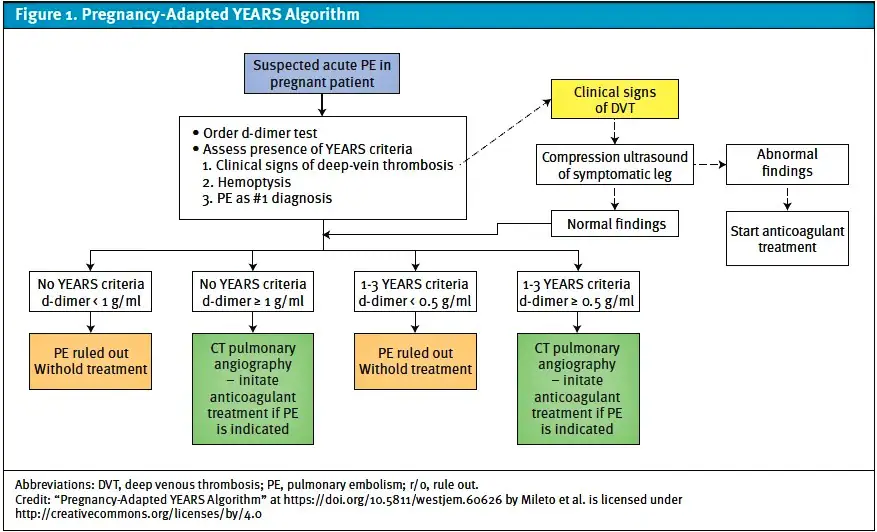
Venous thromboembolism (VTE) is a leading cause of maternal mortality in the United States21 with a prevalence of 0.5-2.0 per 1,000 pregnant women.22,23 The risk of VTE in pregnancy is increased 4-5 fold compared to nonpregnant women with the greatest risk in the third trimester and post-partum periods.22 The majority of VTE in pregnancy are deep vein thromboses (DVT) most commonly occurring in the lower extremities, although upper extremity and ovarian veins are other possible locations. About 20-25% of VTE cases in pregnancy involve a pulmonary embolism (PE).24 VTE results from physiologic changes in pregnancy including hypercoagulability, increased venous stasis, uterine compression of the pelvic veins and inferior vena cava,25 and decreased venous outflow.26 A previous diagnosis of DVT or PE is the greatest risk factor for VTE in pregnancy and is associated with a 3-4 fold increase in risk of recurrent VTE.27 The diagnosis of VTE may include D-dimer and/or imaging tests like lower extremity compression ultrasound, computed tomography (CT) of the chest with intravenous contrast, and/or ventilation perfusion scanning (VQ). The YEARS algorithm incorporates D-dimer testing cut-offs and clinical parameters (signs of DVT, hemoptysis, clinical suspicion for alternative diagnoses) to aid in deciding which patients need chest imaging.28
History
Presenting symptoms of VTE include unilateral lower extremity edema and/or skin discoloration, SOB, hemoptysis, pleuritic chest pain, and syncope. Symptoms of VTE tend to reach maximal intensity over 2-3 days, in contrast to conditions like anemia that have a more insidious onset.29 Delayed presentations are not uncommon. VTE is more likely in pregnant women with a past medical history of VTE, thrombophilia (20-50% of pregnancy related VTE), sickle cell disease, systemic lupus erythematosus, nephrotic syndrome, venous insufficiency, and recent systemic infection.29,30 In addition, antiphospholipid syndrome is the most commonly acquired thrombophilia in pregnancy leading to a more hypercoagulable state.31 Other pregnancy related risk factors include any history of hyperemesis gravidarum, ovarian hyperstimulation syndrome, cesarean delivery of index pregnancy, preeclampsia, and postpartum hemorrhage >1 liter.30,32 Obesity, age >35 years, and parity ≥3 are risk factors that are increasing in prevalence.33
Physical Exam
Findings of VTE can be subtle; the incidence of asymptomatic DVT diagnosed with PE is 30%, while 40-50% of DVTs are associated with asymptomatic PE.34 Tachycardia is the most common physical exam finding in PE. Although research on normal heart rates (HR) in pregnancy are limited, 1 study found HR to be 63-105 beats per minute (BPM) at 12 weeks of gestation, and 68-115 BPM at 34.1 weeks of gestation.35 Jugular venous distention (JVD, defined as point of maximal impulse >4 cm from the sternal angle) may be seen in PE due to increased cardiac output and venous obstruction.36 Additional findings with PE include increased respiratory effort, normal lung sounds, and fever. A unilateral calf circumference discrepancy of at least 2 cm was found in 80% of pregnant women with confirmed DVT.29 In addition to calf circumference discrepancy, symptoms isolated to the left lower extremity and symptom occurrence during the first trimester increase the pre-test probability of DVT.37
Complications of DVT
There is a high proportion of proximal DVTs in the pregnant population.38 Left-sided DVT should raise suspicion for compression of the left common iliac vein between the right common iliac artery and lumbar vertebrae (May Thurner syndrome).38 While uncommon, massive iliofemoral vein thrombosis can result in pitting edema and pallor of the affected extremity (phlegmasia alba dolens). Venous obstruction can rarely lead to compartment syndrome and venous ischemia (phlegmasia cerulea dolens) characterized by tense muscular compartments and tissue cyanosis or poikilothermia.39 Extremity pulselessness, delayed capillary refill, pallor, tense compartments, or pain with passive stretch are signs of limb threatening illness and warrant emergent medical attention. Hypoxia, hypotension, and tachycardia are worrisome for massive PE and also warrant emergent medical attention.
Diagnostic Testing for VTE
In hemodynamically stable pregnant patients with low to moderate pretest probability of PE and DVT, the YEARS algorithm (Figure 1) recommends lower extremity compression ultrasound (CUS) and, if positive, to treat with anticoagulation (low molecular weight heparin) and withhold chest imaging.28 In patients where there is only clinical suspicion for DVT, it is reasonable to first perform CUS, and if the study is negative or non-diagnostic to repeat CUS in 5 to 7 days as infra-popliteal vein thrombi may propagate during this time period.40 Whole leg US is an alternative to CUS demonstrating better detection of infra-popliteal vein thrombi, although the clinical significance is not well studied in pregnancy.40 In patients concerning for PE without signs and symptoms of DVT, the YEARS algorithm recommends a D-dimer test. An elevated D-dimer, or patients with high pretest probability, must be further investigated with diagnostic chest imaging, depending on assay and cut-off threshold. The 2 major imaging modalities include CT and nuclear scintigraphy (VQ) for patients with a normal chest x-ray and no chronic respiratory conditions.29 Although not formally studied, the evaluation of VTE in urgent care may be appropriate for patients without emergent features provided there is reliable access to D-dimer and CUS.
Imaging for PE
About two-thirds of pregnant women require some form of diagnostic chest imaging for the evaluation of PE.40 The European Society of Cardiology (ESC), American College of Obstetrics and Gynecology (ACOG), and American Thoracic Society (ATS) recommend CT or VQ as reasonable tests to exclude PE in pregnant women.29,41,42 A 2017 Cochrane review cautioned that similar pooled rates of nondiagnostic results exist (14% in CT, 12% in VQ),43 although since its publication there has been widespread adoption of CT protocols that adjust contrast dose and administration rate to accommodate changes in maternal blood volume and tachycardia.44, 45 CT is the gold standard for diagnosing PE in pregnant and nonpregnant adults, however the fear of radiation exposure may influence the decision to order a VQ scan.46 Although exposure varies with gestational age and maternal body habitus, the estimated fetal dose of ionizing radiation is 0.01-0.66 mGy and 0.1-0.5 mGy for CT and VQ, respectively.47 These are both well below the threshold of 50 mGy associated with increased risk of fetal anomalies, growth restrictions, and abortion.48 Around 20% of VQ scans in pregnant patients have indeterminate probability, therefore requiring CT imaging and increasing the cumulative fetal and maternal radiation exposure.49 ATS guidelines,42 cited in ESC41 and ACOG29 consensus statements, still recommend VQ, albeit with low certainty due to low quality evidence of maternal risk for breast cancer from CT radiation exposure: “uncertainties are large, and the risk estimates vary widely.”42,50 More recent evidence suggests no significant short-term risk of breast cancer from CT imaging in pregnant women.51
Management
The treatment of VTE in pregnancy varies based on clinical stability and the risk of clinical deterioration. Clinically stable patients with VTE are typically managed in the outpatient setting with low molecular weight heparin. DVT associated with May Thurner syndrome typically requires vascular stenting in addition to anticoagulation.38 High-risk PE is classified as cardiac arrest or obstructive shock. Circulatory compromise is evidenced by hypotension (systolic blood pressure <90 mm Hg or drop of ≥40 mm Hg), right ventricular dilation on CT or echocardiogram, and elevated troponin or B-type natriuretic peptide.41,52 High risk PE and DVT with limb threatening features warrant hospital or intensive care unit admission and may be managed with intravenous thrombolytics or unfractionated heparin, catheter-based thrombolysis, and/or mechanical thrombectomy.41
Urgent Care Approach to SOB in Pregnancy
A full history and physical examination are essential to distinguish emergent pathology from more benign causes of SOB in pregnancy. Urgent care (UC) providers should evaluate for changes in exercise tolerance, peripheral edema, or weight gain. Key physical exam findings indicating cardiopulmonary abnormalities include pulmonary rales or crackles, JVD, pitting edema, or popliteal fossa tenderness. A complete set of vitals should be obtained, including HR, BP, oxygen saturation, respiratory rate, temperature, and fetal heart tones if available. Although HR greater than 100 BPM may be normal after 18 weeks of gestation, new onset or undifferentiated tachycardia warrants urgent investigation (eg, electrocardiogram, urinalysis, evaluation for anemia and thyroid) unless emergent evaluation is otherwise indicated.53,54 Less than 3% of healthy pregnant patients have a SBP less than 95 to 102 mm Hg—a SBP less than 90 mm Hg is worrisome.55 Diagnostic testing in UC should include a complete blood count (evaluate for leukocytosis, thrombocytopenia, new or worsened anemia), urinalysis, electrocardiogram, and chest x-ray. Pregnant patients with SOB accompanied by chest pain, loss of consciousness, dizziness or presyncope, tachycardia (HR >100 BPM), hypotension (SBP <90 mm Hg), hemoptysis, hypoxia, severe edema, popliteal fossa tenderness, and preeclampsia/eclampsia symptoms (headache, visual changes, seizure activity, right upper quadrant pain) or signs (hypertension, new neurologic deficits, hemolysis, transaminitis, proteinuria) should be referred to the ED without delay.6,10,11-12,17-19, 53-55 While awaiting ED transport, it is reasonable to administer treatments in UC such as supplemental oxygen or nebulized breathing treatments. In the absence of emergent features, communicating with a patient’s obstetrician is prudent to coordinate follow-up care, and this may also reveal additional medical concerns not expressed by the patient.
Takeaway Points
- SOB and edema are common symptoms during pregnancy.
- New onset or undifferentiated tachycardia (HR greater than 100) warrant urgent investigation (eg, ECG, urinalysis, evaluation of anemia and thyroid) unless emergent evaluation is otherwise indicated.54
- SBP less than 90 mm Hg defines hypotension in pregnancy.55
- Gestational hypertension is defined as SBP ≥140 mm Hg or DBP ≥90 mm Hg on ≥2 occasions ≥4 hours apart; Severe gestational hypertension is defined as SBP ≥160 mm Hg or ≥110 mm on ≥2 occasions ≥15 minutes apart.17
- The risk of pregnancy related VTE is greatest in the third trimester and postpartum periods.29
- Eighty percent of pregnant women with VTE present with signs and symptoms of DVT; a unilateral calf circumference discrepancy of at least 2 cm strongly suggests a diagnosis of DVT.29
- The YEARS algorithm guides diagnostic testing for PE using D-dimer cutoffs based on symptoms and clinical gestalt.28
- Low molecular weight heparin is the outpatient treatment of choice for VTE in pregnancy.29
Manuscript submitted May 19, 2025; accepted September 3, 2025.
References
- Gilbert R, Auchincloss JH Jr. Dyspnea of pregnancy. Clinical and physiological observations. Am J Med Sci. 1966;252(3):270-276. doi:10.1097/00000441-196609000-00004
- Jensen D, Wolfe LA, Slatkovska L, Webb KA, Davies GA, O’Donnell DE. Effects of human pregnancy on the ventilatory chemoreflex response to carbon dioxide. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1369-R1375. doi:10.1152/ajpregu.00862.2004
- Jensen D, Duffin J, Lam YM, et al. Physiological mechanisms of hyperventilation during human pregnancy. Respir Physiol Neurobiol. 2008;161(1):76-86. doi:10.1016/j.resp.2008.01.001
- Koehler KF, Helguero LA, Haldosén LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev. 2005;26(3):465-478. doi:10.1210/er.2004-0027
- Wu Y, Ye H, Liu J, et al. Prevalence of anemia and sociodemographic characteristics among pregnant and non-pregnant women in southwest China: a longitudinal observational study. BMC Pregnancy Childbirth. 2020;20(1):535. Published 2020 Sep 14. doi:10.1186/s12884-020-03222-1
- American College of O, Gynecologists’ Committee on Practice B-O. Anemia in Pregnancy: ACOG Practice Bulletin, Number 233. Obstet Gynecol. 2021;138(2):e55-e64. doi:10.1097/aog.0000000000004477.
- Jones CE, Jamil Y. Management of asthma in pregnancy. Clin Med (Lond). 2025;25(1):100277. doi:10.1016/j.clinme.2024.100277
- Bravo-Solarte DC, Garcia-Guaqueta DP, Chiarella SE. Asthma in pregnancy. Allergy Asthma Proc. 2023;44(1):24-34. doi:10.2500/aap.2023.44.220077
- Eltawil Y, Callander JK, Loftus PA. Rhinologic Conditions of Pregnancy: A Retrospective Cohort Study. OTO Open. 2025;9(2):e70114. Published 2025 Apr 25. doi:10.1002/oto2.70114
- Dombrowski MP, Schatz M; ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin: clinical management guidelines for obstetrician-gynecologists number 90, February 2008: asthma in pregnancy. Obstet Gynecol. 2008;111(2 Pt 1):457-464. doi:10.1097/AOG.0b013e3181665ff4
- Ashby T, Staiano P, Najjar N, Louis M. Bacterial pneumonia infection in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2022;85(Pt A):26-33. doi:10.1016/j.bpobgyn.2022.07.001
- Influenza in Pregnancy: Prevention and Treatment: ACOG Committee Statement No. 7. Obstet Gynecol. 2024;143(2):e24-e30. doi:10.1097/AOG.0000000000005479
- Obesity in Pregnancy: ACOG Practice Bulletin, Number 230. Obstet Gynecol. 2021;137(6):e128-e144. doi:10.1097/AOG.0000000000004395
- ACOG Practice Bulletin No 156: Obesity in Pregnancy [published correction appears in Obstet Gynecol. 2016 Dec;128(6):1450. doi: 10.1097/AOG.0000000000001807.]. Obstet Gynecol. 2015;126(6):e112-e126. doi:10.1097/AOG.0000000000001211
- Macedo TCC, Montagna E, Trevisan CM, Zaia V, de Oliveira R, Barbosa CP, Laganà AS, Bianco B. Prevalence of preeclampsia and eclampsia in adolescent pregnancy: A systematic review and meta-analysis of 291,247 adolescents worldwide since 1969. Eur J Obstet Gynecol Reprod Biol. 2020 May;248:177-186.
- Battarbee AN, Sinkey RG, Harper LM, Oparil S, Tita ATN. Chronic hypertension in pregnancy. Am J Obstet Gynecol. 2020 Jun;222(6):532-541.
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet Gynecol. 2020;135(6):1492-1495. doi:10.1097/AOG.0000000000003892
- Bauersachs J, König T, van der Meer P, Petrie MC, Hilfiker-Kleiner D, Mbakwem A, Hamdan R, Jackson AM, Forsyth P, de Boer RA, Mueller C, Lyon AR, Lund LH, Piepoli MF, Heymans S, Chioncel O, Anker SD, Ponikowski P, Seferovic PM, Johnson MR, Mebazaa A, Sliwa K. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2019 Jul;21(7):827-843.
- American College of Obstetricians and Gynecologists’ Presidential Task Force on Pregnancy and Heart Disease and Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 212: Pregnancy and Heart Disease. Obstet Gynecol. 2019;133(5):e320-e356. doi:10.1097/AOG.0000000000003243
- Minhas AS, Rahman F, Gavin N, et al. Cardiovascular and Obstetric Delivery Complications in Pregnant Women With Valvular Heart Disease. Am J Cardiol. 2021;158:90-97. doi:10.1016/j.amjcard.2021.07.038
- Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-Related Mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130(2):366-373. doi:10.1097/AOG.0000000000002114
- Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143(10):697-706. doi:10.7326/0003-4819-143-10-200511150-00006
- Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJ. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost. 2008;6(4):632-637. doi:10.1111/j.1538-7836.2008.02921.x
- Blanco-Molina A, Rota LL, Di Micco P, et al. Venous thromboembolism during pregnancy, postpartum or during contraceptive use. Thromb Haemost. 2010;103(2):306-311. doi:10.1160/TH09-08-0559
- Varrias D, Spanos M, Kokkinidis DG, Zoumpourlis P, Kalaitzopoulos DR. Venous Thromboembolism in Pregnancy: Challenges and Solutions. Vasc Health Risk Manag. 2023;19:469-484. Published 2023 Jul 20. doi:10.2147/VHRM.S404537
- Langer AL, Connell NT. Update on pregnancy-associated venous thromboembolism. Thrombosis Update. 2022;8:100107. https://doi.org/10.1016/j.tru.2022.100107
- Pabinger I, Grafenhofer H, Kyrle PA, et al. Temporary increase in the risk for recurrence during pregnancy in women with a history of venous thromboembolism. Blood. 2002;100(3):1060-1062. doi:10.1182/blood-2002-01-0149
- Mileto A, Rossi G, Krouse B, et al. Pregnancy-adapted YEARS Algorithm: A Retrospective Analysis. West J Emerg Med. 2024;25(1):136-143. doi:10.5811/westjem.60626
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 196: Thromboembolism in Pregnancy [published correction appears in Obstet Gynecol. 2018 Oct;132(4):1068. doi: 10.1097/AOG.0000000000002923.]. Obstet Gynecol. 2018;132(1):e1-e17. doi:10.1097/AOG.0000000000002706
- Nelson-Piercy C, MacCallum P, Mackillop L. Green-top Guideline No. 37a – reducing the risk of venous thromboembolism during pregnancy and the puerperium. London, Royal College of Obstetricians and Gynaecologists, 2015
- Khare M, Nelson-Piercy C. Acquired thrombophilias and pregnancy. Best Pract Res Clin Obstet Gynaecol. 2003;17(3):491-507. doi:10.1016/s1521-6934(03)00013-0
- Havers-Borgersen E, Butt JH, Johansen M, et al. Preeclampsia and Long-Term Risk of Venous Thromboembolism. JAMA Netw Open. 2023;6(11):e2343804. doi:10.1001/jamanetworkopen.2023.43804
- Simcox LE, Ormesher L, Tower C, Greer IA. Pulmonary thrombo-embolism in pregnancy: diagnosis and management. Breathe (Sheff). 2015;11(4):282-289. doi:10.1183/20734735.008815
- Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med. 2008;359(19):2025-2033. doi:10.1056/NEJMra0707993
- Green LJ, Mackillop LH, Salvi D, et al. Gestation-Specific Vital Sign Reference Ranges in Pregnancy. Obstet Gynecol. 2020;135(3):653-664. doi:10.1097/AOG.0000000000003721
- Wagner SM, Waldman IN, Karikari KA, Kunselman AR, Smith ER, Deimling TA. The Impact of Pregnancy on the Evaluation of Chest Pain and Shortness of Breath in the Emergency Department. J Acute Med. 2018;8(4):149-153. doi:10.6705/j.jacme.201812_8(4).0002
- Le Moigne E, Genty C, Meunier J, et al. Validation of the LEFt score, a newly proposed diagnostic tool for deep vein thrombosis in pregnant women. Thromb Res. 2014;134(3):664-667. doi:10.1016/j.thromres.2014.07.009
- Schrufer-Poland TL, Florio K, Grodzinsky A, Borsa JJ, Schmidt L. Management of May Thurner Syndrome in Pregnant Patients. J Cardiovasc Dev Dis. 2022;9(12):410. Published 2022 Nov 23. doi:10.3390/jcdd9120410
- Durrani M, Hamidi A, Lampley C, Dasgupta S. Catheter directed thrombolysis of Phlegmasia Cerulea Dolens: A case report. JEM Reports. 2023;(2)1. doi.org/10.1016/j.jemrpt.2023.100010
- Bellesini M, Robert-Ebadi H, Combescure C, Dedionigi C, Le Gal G, Righini M. D-dimer to rule out venous thromboembolism during pregnancy: A systematic review and meta-analysis. J Thromb Haemost. 2021;19(10):2454-2467. doi:10.1111/jth.15432
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543-603. doi:10.1093/eurheartj/ehz405
- Leung AN, Bull TM, Jaeschke R, et al. American Thoracic Society documents: an official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline–Evaluation of Suspected Pulmonary Embolism in Pregnancy. Radiology. 2012;262(2):635-646. doi:10.1148/radiol.11114045
- van Mens TE, Scheres LJ, de Jong PG, Leeflang MM, Nijkeuter M, Middeldorp S. Imaging for the exclusion of pulmonary embolism in pregnancy. Cochrane Database Syst Rev. 2017;1(1):CD011053. Published 2017 Jan 26. doi:10.1002/14651858.CD011053.pub2
- Mehdipoor G, Jimenez D, Bertoletti L, et al. Imaging modalities for confirming pulmonary embolism during pregnancy: results from a multicenter international study. Eur Radiol. 2022;32(2):1238-1246. doi:10.1007/s00330-021-08161-9
- Picone C, Fusco R, Tonerini M, et al. Dose Reduction Strategies for Pregnant Women in Emergency Settings. J Clin Med. 2023;12(5):1847. Published 2023 Feb 25. doi:10.3390/jcm12051847
- Tromeur C, van der Pol LM, Le Roux PY, et al. Computed tomography pulmonary angiography versus ventilation-perfusion lung scanning for diagnosing pulmonary embolism during pregnancy: a systematic review and meta-analysis. Haematologica. 2019;104(1):176-188. doi:10.3324/haematol.2018.196121
- Tremblay E, Thérasse E, Thomassin-Naggara I, Trop I. Quality initiatives: guidelines for use of medical imaging during pregnancy and lactation. Radiographics. 2012;32(3):897-911. doi:10.1148/rg.323115120
- Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation [published correction appears in Obstet Gynecol. 2018 Sep;132(3):786. doi: 10.1097/AOG.0000000000002858.]. Obstet Gynecol. 2017;130(4):e210-e216. doi:10.1097/AOG.0000000000002355
- Sadeghi S, Arabi Z, Moradi M, Raofi E. Diagnostic imaging to investigate pulmonary embolism in pregnancy using CT-Pulmonary angiography versus perfusion scan. J Res Med Sci. 2021;26:37. Published 2021 Jun 30. doi:10.4103/jrms.JRMS_113_20
- National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. 2006;Washington, DC: The National Academies Press. Retrieved 27 March 2025 from https://www.philrutherford.com/Radiation_Risk/BEIR/BEIR_VII.pdf.
- Burton KR, Park AL, Fralick M, Ray JG. Risk of early-onset breast cancer among women exposed to thoracic computed tomography in pregnancy or early postpartum. J Thromb Haemost. 2018;16(5):876-885. doi:10.1111/jth.13980
- Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of Pulmonary Embolism During Pregnancy: A Multicenter Prospective Management Outcome Study. Ann Intern Med. 2018;169(11):766-773. doi:10.7326/M18-1670
- Centers for Disease Control and Prevention. Hear Her: Recognizing Urgent Maternal Warning Signs. Updated 2023. https://www.cdc.gov/hearher. Accessed June 17, 2025.
- Coad F, Frise C. Tachycardia in pregnancy: when to worry?. Clin Med (Lond). 2021;21(5):e434-e437. doi:10.7861/clinmed.2021-0495
- Green LJ, Kennedy SH, Mackillop L, et al. International gestational age-specific centiles for blood pressure in pregnancy from the INTERGROWTH-21st Project in 8 countries: A longitudinal cohort study. PLoS Med. 2021;18(4):e1003611. Published 2021 Apr 27. doi:10.1371/journal.pmed.1003611
Author Affiliation: John Ramos, MMS, PA-C, CAQ-EM, Duke University Hospital, Department of Emergency Medicine. Author has no relevant financial relationships with any ineligible companies.
