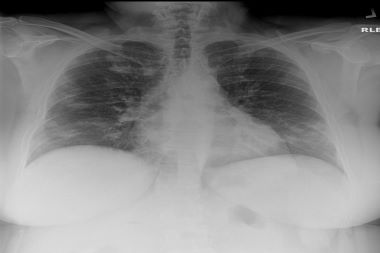
Even with a U.S. death toll in the hundreds of thousands, some people remain skeptical of vaccines, the value of social distancing, and the overall dangers of COVID-19. The CBS affiliate in Dallas ran a story recently that might make a dent, however. It quotes a trauma surgeon and assistant professor at Texas Tech University as saying that survivors are likely to have some degree of lung damage after recovering—with their x-rays sometimes worse than …
Read More