Published on
Download the article PDF: Herpes Simplex Virus Infections An Overview Of Testing For The Urgent Care Clinician
Urgent Message: Polymerase chain reaction testing is recommended for patients with lesions that could represent herpes simplex virus infection. It is imperative that urgent care clinicians understand the utility and characteristics of such testing as well as the implications of findings.
Brittney Tice, FNP, DNP; Joseph Something, PA; Benjamin Zimmerman, PhD
Editor’s Note: The patient case scenario is hypothetical.
Abstract
In the urgent care (UC) setting, patients commonly present with nominal requests for herpes simplex virus (HSV) testing. HSV infections are common, pleomorphic, and associated with significant stigma. This combination creates a situation where decisions regarding which, if any, test(s) to obtain can be highly impactful for the mental health of patients and their romantic partners. It is imperative that UC clinicians understand the utility and test characteristics of HSV testing and the implications of findings before ordering testing. Given that both HSV-1 and HSV-2 are chronic infections, serologic testing results have the potential for lifelong consequences and should only be obtained in settings where clinically indicated and with appropriate patient counseling.
Clinical Scenario
A 42-year-old man presented to UC requesting a “blood test for herpes.” The patient denied genital lesions, prodromal symptoms, or other genitourinary (GU) complaints. Upon further questioning, the patient stated he had concern for HSV-2 specifically after finding out that his partner had tested positive for HSV-2 by serology. His vital signs, general appearance, and GU exam were normal. The clinician evaluating him agreed to order a serologic immunoglobulin G (IgG) test for HSV. The results returned with a slightly elevated HSV-2 IgG titer. When the patient was called the following day with test results, he expressed significant anxiety and had many questions about the meaning of his test results.
Introduction
HSV-1 and HSV-2 are 2 of the 8 members of the Herpesviridae family that infect humans (Table 1). This family also includes varicella-zoster virus (VZV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), and human herpesviruses 6-8.[1],[2] The herpesvirus family causes a wide range of infections with distinct clinical manifestations, but this article will focus on considerations for testing for HSV-1 and HSV-2 in patients presenting to UC.[3]
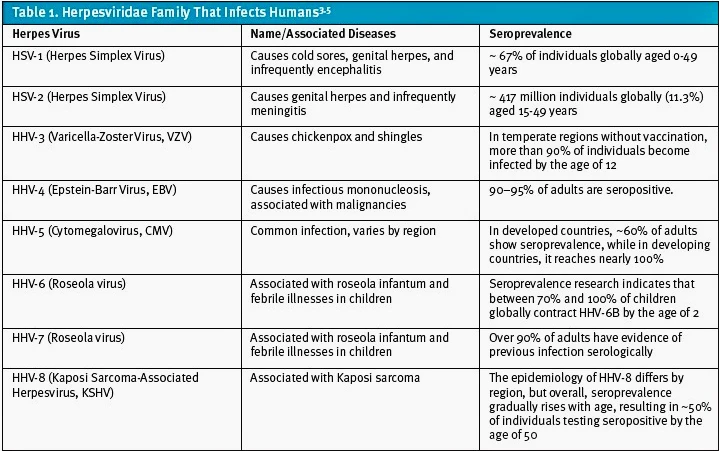
HSV-1 and HSV-2 infections are highly prevalent worldwide with over 4.2 billion people infected. HSV-1 is more common with an estimated 3.7 billion people living with the virus compared to an estimated 490 million living with HSV-2.[4] Genital infections specifically are more commonly caused by HSV-2 but can be caused by either virus.[5] In a 2020 study looking at 15-49 year olds, HSV-1 was estimated to be responsible for approximately 40% of genital HSV (gHSV) infections.5 For both HSV-1 and HSV-2, the prevalence was found to be higher in females.5
In the United States, there are roughly 96 million people believed to be chronically infected with HSV-1 that are in the age group of 14-49 years old.[6] For the same age group, there are 24.2 million people infected with HSV-2. Additionally, HSV-1 affects about 48% of Americans overall and is typically associated with oral lesions. In contrast to HSV-1, HSV-2 rarely will cause orolabial infection and primarily affects the genital and anorectal areas. Approximately 12% of Americans have chronic HSV-2 infection.[7]
In UC settings, asymptomatic patients commonly request “herpes testing,” usually referring to serologic testing in the absence of active lesions. Patients are most commonly concerned with HSV-2 testing due to the stigma surrounding the virus.[8] It is the UC clinician’s responsibility to ensure patients understand the limitations of HSV-2 testing and when it may have clinical utility. The goal of this article is to clarify for clinicians when serologic testing is and is not recommended, especially as testing without appropriate indication can be harmful.
Background
HSV-1 and HSV-2 are common and chronic. There are rare, serious medical consequences associated with HSV infections, however, infection commonly has considerable impact on the mental health of those afflicted.[9] The prevalence of HSV-2 infection in UC environments has not been specifically studied. However, when studied in the urban emergency department setting in Baltimore, the seroprevalence of HSV-2 was high (~54%).[10] This suggests that particularly in urban settings, there is a critical need for guidelines around targeted testing and treatment.
While the rates of HSV-2 appear to be declining,[11] the true seroprevalence of HSV-2 is difficult to know for certain as current guidelines do not recommend routine serologic testing, and many patients are believed to be asymptomatically infected.[12] HSV-2 prevalence varies based on demographics, sexual practices, and underlying co-morbidities. The prevalence of HSV-2 is higher in women and those co-infected with human immunodeficiency virus (HIV).[13],[14]
Manifestations of HSV Infection
After initially infecting epithelial cells, HSV will typically transition to a latent state residing in the ganglia of peripheral neurons.1 Both HSV-1 and HSV-2 can be transmitted even in the absence of active skin lesions due to a phenomenon known as “asymptomatic viral shedding.”[15],[16]
Most individuals with both HSV-1 and HSV-2 infection are asymptomatic. However, when symptoms are present, HSV-1 and HSV-2 can cause recurrent lesions of the oral or genital region.15,16 HSV-1 is typically linked to oral infections commonly referred to as “cold sores” or “fever blisters.” However, HSV-1 can also cause genital herpes (gHSV) in cases of oral-genital contact. Historically, orolabial herpes infection was typically attributed to HSV-1, whereas genital infection was attributed to HSV-2. In recent years, however, HSV-1 has become increasingly recognized as a cause of urogenital herpes infection.[17] HSV-1 is becoming more common on the genitals, especially for young women and men who have sex with men.16
The incubation period of both HSV-1 and HSV-2 is 4-7 days.[18] The lesions can be polymorphic, but in the classic presentation, they progress from flat spots and raised bumps to blisters, pustules, and ulcers, and the lesions can be very painful.15 Skin ulcers eventually form scabs, while oral ulcers remain exposed due to the moist environment.15,16 For the initial infection, the lesions will take approximately 2-3 weeks to resolve, but in cases of recurrence, the lesions will usually resolve in about 5-10 days.18
HSV-1 typically manifests as orolabial lesions with most patients having rare outbreaks. Therefore, serologic testing for HSV-1 is not recommended by the Centers for Disease Control (CDC) or the United States Preventive Services Task Force (USPSTF).11,16 HSV-1 related genital infections have lower rates of symptomatic reactivations than HSV-2. Within the first year, approximately 20-50% of people with HSV-1 will have a symptomatic recurrence compared to >70% in patients with genital HSV-2 infections.18 Individuals with HSV-2 also will typically have more recurrences than those with HSV-1. The median number of symptomatic recurrences within the first year is 1.3 for HSV-1 and 4 for HSV-2.18 For both HSV-1 and HSV-2 genital herpes, recurrences tend to decrease in frequency over time, but many patients continue to experience them for over a decade.18
Patients with HSV infections should be educated about asymptomatic viral shedding and the attendant risks for transmission—which is highest during the first 12 months following initial infection—especially for those with HSV-2.16 Given the low risk for antiviral treatments for HSV, suppressive daily treatment is reasonable to not only reduce frequency and severity of outbreaks but also to reduce frequency of asymptomatic viral shedding. This consideration is especially relevant for patients in sexual relationships that are serodiscordant for HSV-2 (ie, partner is not infected with the same HSV serotype). Daily suppressive antiviral therapy, while helpful, may only decrease the risk of infecting a partner by about 50%.18 Most transmissions of HSV-2 are believed to occur when the infected person is asymptomatically shedding the virus.18,[19]
Overview of Testing
Either type of HSV can be diagnosed by either identification of the virus from lesions or by detection of serum antibodies (ie, serology).[20] Diagnostic testing has evolved over time from less reliable methods such as viral cultures and Tzanck preparations toward more modern serological testing and nucleic acid amplification tests (NAAT). Polymerase chain reaction (PCR) is the most commonly applied type of NAAT test.[21] Importantly, despite common patient requests and clinician orders for HSV serologies, the USPSTF advises against routine serologic screening for HSV-1 and 2 infection in asymptomatic adolescents and adults, including pregnant individuals, due to the inaccuracy and cross-reactivity of these tests and the relatively clinically innocuous nature of HSV infections.11
Viral Testing
For patients presenting with lesions, direct testing from lesions is recommended strongly over serological methods because serology cannot distinguish between active and past infections. Additionally, IgG serology testing will be negative in the weeks following an initial infection.16 Approaches to the direct testing of HSV lesions include viral culture and PCR. In viral culture, the sample is inoculated onto live cells in a laboratory that are observed for cytopathic effects.18 On the other hand, PCR detects the presence of viral DNA by amplifying genetic material from the sample.[22] Both methods require proper sample collection techniques, which involve swabbing or “deroofing” a lesion.
To properly deroof and collect a sample, follow these steps:
1. Choose an unbroken vesicle that is filled with fluid.
2. Clean the area with sterile water or saline, avoiding alcohol or other skin disinfectants.
3. Use a sterile beveled hypodermic needle or a disposable scalpel to gently deroof the vesicle.
4. Collect the sample with the appropriate swab.
5. Place the swab in a viral transport medium and keep at 4°C and ensure arrival at the laboratory within 48 hours of collection.22
Failure to adequately deroof a vesicle can lead to false negative PCR results. Unfortunately, the 2 largest laboratory services in the United States (Labcorp and Quest) do not provide in-depth descriptions of the vesicle swabbing technique.[23],[24]
PCR testing has become the preferred method for HSV DNA detection due to its higher sensitivity and faster turnaround time compared to viral culture.16 Viral culture also requires live viruses, increasing the probability of false negatives. PCR also can more reliably differentiate between HSV-1 and HSV-2 infections.20
Serologic Testing
In patients without active lesions, serological testing is required if electing to pursue testing for HSV infection.16 Serologic tests detect antibodies in response to HSV infection, which gives information about the likelihood of past exposures. However, asymptomatic testing is only recommended in a narrow range of patients: those with either a known seropositive partner or those who have risk factors that predispose them to central nervous system or systemic infection.16
Serologic testing can be categorized into type-specific and type-common testing. Type-specific testing is a serologic assay diagnostic method that detects antibodies targeting specific types or strains of a pathogen. An example of this is the Western blot assay, which is the gold standard for serologic testing, but has limited clinical availability.16 In contrast, type-common antibody testing identifies antibodies but is less specific for differentiating the responsible pathogen. Enzyme-linked immunosorbent assay (ELISA) is typically the type-common testing method when an IgG and immunoglobulin M (IgM) serologic test is ordered and is far more commonly clinically available.
Types of Immunoglobulins: IgM vs IgG
IgM is typically the first antibody produced by the immune system upon initial exposure to an infection. In the context of HSV, IgM antibodies can appear within 3-4 days after the primary infection.[25] However, IgM testing for HSV is rarely recommended due to significant limitations in its interpretability. First, IgM tests are considered type-common and cannot distinguish between HSV-1 and HSV-2 infections.16 IgM antibodies may also be detected during recurrent episodes or due to cross-reactivity with other herpesviruses, leading to possible misinterpretation. Finally, while IgM antibodies appear quickly, the levels also decline after a few weeks, further limiting any clinical utility of this assay in UC settings.25
In contrast, IgG antibodies are produced more gradually and do not reliably appear until at least 2 weeks after infection. IgG antibodies remain detectable for years after an initial infection.21 It is this long-term persistence of IgG antibodies that allows for the identification of past infections, even in asymptomatic patients. Additionally, type-specific IgG tests are preferred, given that they can distinguish between HSV-1 and HSV-2 to certain degrees. This is achieved by targeting glycoproteins which are more specific to each virus type, however, cross-reactivity can also occur.18
ELISA vs Western Blot
ELISA is a widely used serological testing methodology used for detection of various antibodies to infectious agents and in cases of suspected autoimmune disease. While convenient and widely available, ELISA serology testing has important limitations. With regard to HSV testing specifically, multiple studies have shown that ELISA tests for HSV-2 can have high sensitivity but relatively low specificity, leading to false-positive results, especially in low-prevalence populations. Reported sensitivities for HSV-1 ELISA testing ranges from 69-99%, while specificities have been reported between 77-97.8%.[26],[27],[28] HSV-2 results using ELISA have even lower accuracy with sensitivities around 92% and specificity as low as 57.4%.28 This means that many patients with HSV-1—the much more commonly encountered infection—will have false positive HSV-2 ELISA results. This underscores the importance of understanding and avoiding indiscriminate use of the HSV IgG serology among asymptomatic patients.
In Western blot assays, HSV-1 or HSV-2 viral proteins extracted from a patient sample are separated based on protein molecular weights using a technique called gel electrophoresis.20 Western blot is considered the gold standard for HSV antibody detection and is used as the reference standard for determining sensitivity and specificity of other tests.26,28,[29] Due to its higher specificity than ELISA IgG testing, Western blot is recommended for confirming ambiguous or low-titer level “positive” ELISA results, especially for HSV-2. However, HSV Western blot is not commercially available in the United States, and testing is only available through specialized laboratories. As of this publication, for example, the HSV-2 Western blot test is only available through a private laboratory at the University of Washington.[30]
Overall, it is important that patients are aware of the limitations of serological testing with ELISA, most notably the risk for false positives, especially with low index values. A 2016 review of 17 studies indicated that serologic screening tests for HSV-2 showed a false-positive rate of 50% in populations with similar prevalence to U.S. adults. The review article also found that these false positive results can contribute to depression and anxiety among affected individuals.12 Confirmatory testing with Western blot is therefore recommended in cases with positive HSV-2 titers. Given the potential lifelong implications, this recommendation makes sense but is likely to be practically challenging given limited access to Western blot (ie, the recommended confirmatory test). Additionally, comprehensive counseling is recommended after a positive test result is returned.16 Western blot results may require many weeks to return. However, patients can be educated to return to the clinic if they develop lesions, which can be tested by viral PCR swab as previously discussed.21 This complexity in possible testing outcomes and need for potentially extensive counseling underscores the importance of conscientiously approaching HSV serology testing requests, especially from time- and resource-limited UC centers.
Recommendations For Testing
The CDC and the European Centre for Disease Prevention and Control (ECDC) have similar guidelines for HSV serologic testing.10,16
Viral testing for HSV-2 is recommended over serologic testing when lesions are present.16
In 2017, more comprehensive HSV testing guidelines were published in the International Journal of STD and AIDS.10 These authors suggest that NAAT laboratory confirmation (via lesion swabbing) is advised in suspected cases of HSV, regardless of whether the clinical suspicion is high or low. If it is a first suspected outbreak of HSV, swabbing for PCR allows for determination whether the outbreak is related to HSV or another cause and can also distinguish between HSV-1 and HSV-2. Routine serologic testing is discouraged due to the low accuracy of these tests and significant psychosocial consequences of inaccurate results. For these reasons, HSV type-specific serology is only recommended for certain groups and situations, and with low-level evidence (IV, C).10 A detailed discussion of these specific groups and situations is outside of the scope of this article.
The USPSTF similarly recommends against routine asymptomatic serologic testing for HSV in adults and adolescents, regardless of pregnancy status.[31] However, they do make note that serological testing is appropriate for individuals who are in sexual relationships with a partner known to have HSV infection to determine if the pair are serodiscordant.31
The CDC guidelines also advocate for type-specific HSV-2 serologic testing in patients who are (or are believed to be) serodiscordant from their partner. The CDC also recommends that, if collecting an HSV viral swab, a serologic test for syphilis should also be collected because lesions from HSV and syphilis may mimic one another and clinically distinguishing them is not reliable.21
Patients Presenting With Lesions
As previously discussed, NAAT/PCR is recommended in patients presenting with genital or orolabial lesions. NAATs can detect HSV from genital ulcers or other mucocutaneous lesions.
Fortunately, viral NAAT testing has become increasingly available over recent years.16 NAAT testing with proper technique is highly sensitive (90.9-100%) and nearly 100% specific.16
Viral culture is no longer recommended unless no other testing is available because it requires more time and has lower sensitivity.16,20 Serum HSV PCR tests are not recommended to diagnose genital herpes infection unless there is a concern about disseminated infection or visceral involvement (eg, hepatitis).16
Patients Presenting Without Lesions
Given that HSV is a chronic infection without active lesions present most of the time, patients can commonly present requesting HSV testing while asymptomatic. In such patients, a thorough understanding of the characteristics of serologic testing modalities is crucial. Given the nuances previously discussed, it is unsurprising that both clinicians and patients are commonly confused about the interpretation of serologic testing for HSV.20
Serology testing can include ELISA and Western blot, however, only ELISA is commercially available at this time in the United States. These tests work by detecting HSV glycoproteins or HSV-specific antibodies.20 Older serologic testing methods for IgG and IgM antibodies are less reliable than ELISA and rely on highly cross-reactive whole-virus antigens and therefore are not recommended for use.18
IgM serology testing is not recommended because it is a type-common test that cannot differentiate between HSV-1 and HSV-2 and IgM antibodies are not enduring in the same way IgG antibodies persist. Furthermore, HSV IgG serologic testing is also discouraged except in specific situations (eg, serodiscordant couples) due to its poor ability to distinguish between HSV-1 and 2 infections and the site of infection. HSV-2 cross reactivity in patients with HSV-1 infection (ie, false positives) is a common phenomenon, and Western blot confirmatory testing is largely inaccessible. Therefore, given potential psychological or social harms to patients and need for extensive counseling, it is sensible to explain to patients the hazards of HSV serology testing and defer the testing to clinics specializing in sexually transmitted infections.16
In addition to the CDC, the USPSTF, the American Academy of Family Physicians (AAFP), and the American College of Obstetricians and Gynecologists (ACOG) also provide recommendations regarding HSV testing. The USPSTF, AAFP, and ACOG all agree that routine HSV testing in asymptomatic individuals should not be performed. Patients can also be referred to the respective guidelines to facilitate discussions with their romantic partner(s).
Takeaway Points
- PCR/NAAT testing is recommended in patients with lesions that could represent HSV infection. These tests have good sensitivity and are very specific for each HSV subtype. Lesions must be unroofed before obtaining a PCR swab for the test to be accurate.
- Routine HSV-1 and/or 2 serology should not be included in testing for patients presenting for routine STI screening. IgM serology is not recommended in any scenario, and IgG testing should only be performed in specific scenarios of high risk and after appropriate counseling.
- Any time an HSV viral lesion swab is collected, a serologic syphilis test is also recommended because clinically distinguishing HSV from syphilis is otherwise not reliable.
- HSV-2 serology is reasonable in patients with concerns for serodiscordance in their romantic relationships. Patients in high-risk relationships should be educated on transmission risk and asymptomatic viral shedding.
Manuscript submitted October 11, 2024; accepted May 1, 2025.
References
- [1]. Gustavsson E, Grünewald K, Elias PE, Hällberg BM. Dynamics of the Herpes simplex virus DNA polymerase holoenzyme during DNA synthesis and proof-reading revealed by Cryo-EM. Nucleic Acids Res. 2024; gkae374. doi:[10.1093/nar/gkae374].
- [2]. International Committee on Taxonomy of Viruses (ICTV). Virus taxonomy: 2020 release. Published 2024. Accessed March 24, 2024. Available from: https://ictv.global/taxonomy/
- [3]. Bharucha T, Houlihan CF, Breuer J. Herpesvirus infections of the central nervous system. Semin Neurol. 2019;39(3):369-382. doi:10.1055/s-0039-1687837.
- [4]. James C, Harfouche M, Welton NJ, et al. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020;98(5):315-329. doi:10.2471/BLT.19.237149.
- [5]. Harfouche M, AlMukdad S, Alareeki A, et al. Estimated global and regional incidence and prevalence of herpes simplex virus infections and genital ulcer disease in 2020: mathematical modelling analyses. Sex Transm Infect. Published online December 10, 2024. doi:10.1136/sextrans-2024-056307.
- [6]. National Center for Health Statistics (NHANES). 2015-2016 Overview. Accessed from: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overview.aspx?BeginYear=2015.
- [7]. Crawford KHD, Selke S, Pepper G, et al. Performance characteristics of highly automated HSV-1 and HSV-2 IgG testing. J Clin Microbiol. 2024;62:e00263-24. doi:10.1128/jcm.00263-24.
- [8]. Wang C, Xu Y, Zhang X, et al. Advances in herpes simplex virus diagnostics: from laboratory to point-of-care testing. Front Microbiol. 2023;14:1123456. doi:10.3389/fmicb.2023.1123456.
- [9]. You S, Yaesoubi R, Lee K, et al. Lifetime quality-adjusted life years lost due to genital herpes acquired in the United States in 2018: a mathematical modeling study. Lancet Reg Health Am. 2023;19:100427. doi:10.1016/j.lana.2023.100427.
- [10]. Patel R, Kennedy OJ, Clarke E, et al. 2017 European guidelines for the management of genital herpes. Int J STD AIDS. 2017;28(14):1366-1379. doi:10.1177/0956462417727194.
- [11]. Asher G, Feltner C, Harrison W, et al. Serological screening for genital herpes: a reaffirmation evidence update for the U.S. Preventive Services Task Force. Evid Synth No. 224. Agency for Healthcare Research and Quality; 2023. AHRQ Publication No. 22-05296-EF-1.
- [12]. Feltner C, Grodensky C, Ebel C, et al. Serological screening for genital herpes: an evidence review for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality (US); 2016 Dec. (Evidence Syntheses, No. 149.)
- [13]. Freeman EE, Weiss HA, Glynn JR, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73-83.
- [14]. Looker KJ, Welton NJ, Sabin KM, et al. Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: a population attributable fraction analysis using published epidemiological data. Lancet Infect Dis. 2020;20(2):240-249.
- [15]. Kimberlin DW, Rouse DJ. Genital herpes. N Engl J Med. 2004;350(19):1970-1977.
- [16]. Workowski KA, et al. Sexually transmitted infections treatment guidelines, 2021. Accessed from: https://www.cdc.gov/std/treatment-guidelines/default.htm.
- [17]. Falk-Hanson E, Marconi A, Sarrouf EB, Sullivan P. Herpes Simplex Type 1 as the Predominant Cause of Genital Herpes in College Students. Sex Transm Dis. 2024;51(12):784-787. doi:10.1097/OLQ.0000000000002060. Epub 2024 Aug 8. PMID: 39102507.
- [18]. Gnann JW Jr, Whitley RJ. Genital herpes. N Engl J Med. 2016;375(7):666-674.
- [19]. Schiffer JT, Mayer BT, Fong Y, Swan DA, Wald A. Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding. J R Soc Interface. 2014;11(95):20140160. doi:10.1098/rsif.2014.0160.
- [20]. Nath P, Kabir MA, Doust SK, Ray A. Diagnosis of herpes simplex virus: laboratory and point-of-care techniques. Infect Dis Rep. 2021;13(2):518-539. doi:10.3390/idr13020049.
- [21]. Miller JM, Binnicker MJ, Campbell S, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update. Clin Infect Dis. 2018;67(6):e1-e94. doi:10.1093/cid/ciy381.
- [22]. Singh A, Preiksaitis J, Ferenczy A, Romanowski B. The laboratory diagnosis of herpes simplex virus infections. Can J Infect Dis Med Microbiol. 2005;16(2):92-98. doi:10.1155/2005/318294. PMID: 18159535; PMCID: PMC2095011.
- [23]. Labcorp. Herpes simplex virus (HSV) types 1 and 2, NAA. Labcorp website. https://www.labcorp.com/tests/188056/herpes-simplex-virus-hsv-types-1-and-2-naa. Accessed March 10, 2025.
- [24]. Quest Diagnostics. SureSwab Herpes Simplex Virus Type 1 and 2, mRNA, TMA. Quest Diagnostics website. https://testdirectory.questdiagnostics.com/test/test-detail/90570/sureswab-herpes-simplex-virus-type-1-and-2-mrnatma?cc=MASTER. Accessed March 10, 2025.
- [25]. Hsueh PR, Huang LM, Chen PJ, Kao CL, Yang PC. Clin Microbiol Infect. 2004;10(12):1062-1066. doi:10.1111/j.1469-0691.2004.01009.x.
- [26]. Mark HD, Nanda JP, Roberts J, et al. Performance of Focus ELISA tests for HSV-1 and HSV-2 antibodies among university students with no history of genital herpes. Sex Transm Dis. 2007;34(9):681-685. PMCID: PMC2648390.
- [27]. Ashley-Morrow R, Nollkamper J, Robinson NJ, Bishop N, Smith J. Performance of Focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin Microbiol Infect. 2004;10(6):530-536. PMID: 15191381.
- [28]. Agyemang E, Le QA, Warren T, et al. Performance of commercial enzyme-linked immunoassays for diagnosis of herpes simplex virus-1 and herpes simplex virus-2 infection in a clinical setting. Sex Transm Dis. 2017;44(12):763-767.
- [29]. Martins TB, Welch RJ, Hill HR, Litwin CM. Comparison of a multiplexed herpes simplex virus type-specific immunoglobulin G serology assay to immunoblot, Western blot, and enzyme-linked immunosorbent assays. Clin Vaccine Immunol. 2009;16(1):55-60. PMCID: PMC2620677.
- [30]. Hussein A. Analyzing trends in HSV Western Blot results at a reference laboratory. Published 2021. Accessed from: https://digital.lib.washington.edu/researchworks/handle/1773/47495.
- [31]. US Preventive Services Task Force, Mangione CM, Barry MJ, et al. Serologic Screening for Genital Herpes Infection: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA. 2023;329(6):502-507. doi:10.1001/jama.2023.0057
Author Affiliations: Brittney Tice, FNP, DNP, Legacy GoHealth Urgent Care, Portland, Oregon. Joseph Something, PA, Prism Health, Portland, Oregon. Benjamin Zimmerman, PhD, National University of Natural Medicine, Portland, Oregon. Authors have no relevant financial relationships with any ineligible companies.
Read More
- The Urgent Need for STI Testing in Urgent Care Centers
- Understanding the Benefits and Risks of Direct-to-Consumer Testing for Urgent Care
- STDs: Assessment and Treatment in Urgent Care
