Published on
Citation: Hunt E, Greger M, Shipley N. Brief Report: PrEPare for Action – A Quality Improvement Project for Expanding HIV Screening in the Urgent Care Setting During the COVID-19 Pandemic. J Urgent Care Med. 2025; 19(9): 21-23
Download the Article PDF: Brief Report: PrEPare for Action – A Quality Improvement Project for Expanding HIV Screening in the Urgent Care Setting During the COVID-19 Pandemic
Urgent Message: A quality improvement program demonstrated the successful integration of HIV services into urgent care, achieving a higher consent rate than traditional settings.
Erin Hunt, PA; Megan Greger, PA; Neal Shipley MD, MBA, FACEP
Abstract
Introduction: The COVID-19 pandemic caused significant disruptions in human immunodeficiency virus (HIV) screening and prevention in the United States, leading to declines in diagnoses and preexposure prophylaxis (PrEP) prescriptions. To address this issue, a quality improvement program was launched in urgent care centers in New York City to explore their role in HIV screening and PrEP counseling. The goal was to improve access, reduce barriers, and lower HIV transmission by integrating these services into urgent care.
Methods: The program involved 2,800 rapid HIV tests distributed across 17 urgent care sites in the boroughs of Manhattan, Brooklyn, and Queens. Staff were trained in point-of-care testing and risk assessments using inclusive language. Adult patients were offered free HIV screening. Those who tested negative for HIV but were deemed to be high risk for contracting HIV were referred for PrEP. Preliminary positive HIV test results were referred for specialist consultation and confirmatory testing. Data collection was embedded in the electronic medical record system, and throughput times were tracked to evaluate operational efficiency.
Results: From April 2023 to May 2024, 17,439 patients were approached with a 9.2% testing consent rate. Nineteen preliminary positives resulted in 2 confirmed cases. Additionally, 11% of participants were referred for PrEP services. The false positive rate was within the expected range at 1.1%, mainly due to sample processing issues. Evaluation of throughput times indicated no impact on efficiency.
Conclusion: This program demonstrates the success of integrating HIV services into urgent care, achieving a higher consent rate than traditional settings. The findings highlight the crucial role of urgent care centers in expanding access to HIV prevention and surveillance.
Introduction
During the COVID-19 pandemic, human immunodeficiency virus (HIV) screening and prevention efforts in the United States were significantly impacted with a 32% decrease in HIV diagnoses and a 6% drop in pre-exposure prophylaxis (PrEP) prescriptions in 2020 compared to the previous year.1 As traditional healthcare settings faced challenges, urgent care centers saw a surge in patient volumes, serving as a crucial alternative for care.
To evaluate the use of urgent care facilities for HIV screening and PrEP counseling, a quality improvement program was developed with aims to enhance accessibility, reduce barriers, and contribute to lowering HIV transmission rates. This study examines the feasibility and impact of integrating HIV prevention and surveillance in the urgent care setting.
Methods
A total of 2,800 HIV-1/2 Ab/Ag rapid tests from Abbott Rapid Diagnostics were distributed evenly among 17 Northwell Health – GoHealth Urgent Care facilities located across the New York City boroughs of Manhattan, Brooklyn, and Queens. Clinical teams were trained to conduct point-of-care (POC) HIV testing and administer HIV risk assessments using inclusive and nonjudgmental language.
All patients aged 18 years and older seeking urgent care services were offered HIV screening as a supplementary service at no additional cost during the triage process. HIV risk stratification was performed for all patients consenting to screening. Individuals were classified as high risk for contracting HIV compared to the general population if they met any of the following criteria: engaging in intravenous drug use; identifying as a man who has sex with men; having multiple sexual partners; having a sexual partner with HIV who has a detectable viral load; having a history of any sexually transmitted infection (STI) within the past 6 months; or engaging in unprotected vaginal or anal intercourse outside of a monogamous relationship. Individuals testing negative but deemed high risk for contracting HIV received counseling and referrals for PrEP services. Those testing as preliminarily positive underwent confirmatory HIV testing, and if confirmed, were directed to an infectious disease (ID) specialist for further evaluation and treatment.
Data collection was facilitated through tailored templates within the electronic medical record (EMR) system. Door-to-door (D2D) times (ie, total time patients were in the urgent care center) were monitored to evaluate operational efficiency. We compared D2D times across the 17 centers enrolled in the quality improvement program (test sites) to those at the remaining 44 centers within the Northwell Health – GoHealth Urgent Care market (control sites) during the same period. Data was analyzed using an independent 2-sample t-test to determine whether any changes in D2D times between the 2 groups were statistically significant. A p-value of <0.05 was considered significant.
Institutional review board approval was not sought for this quality improvement project as it did not fall under the definition of human subject research. Data was collected and analyzed as a pre-planned aspect of the quality improvement project.
Results
The quality improvement project ran from April 2023 through May 2024. HIV screening was offered to 17,439 patients, with 1,596 (9.2%) opting in to testing. Nineteen patients tested preliminarily positive; individuals who were confirmed HIV positive (0.1%) were referred for further evaluation and treatment. Of those testing negative, 175 individuals (11%) were identified as high risk and were referred for PrEP services. The false positive rate was within the expected range at 1.1%. (Figure 1)
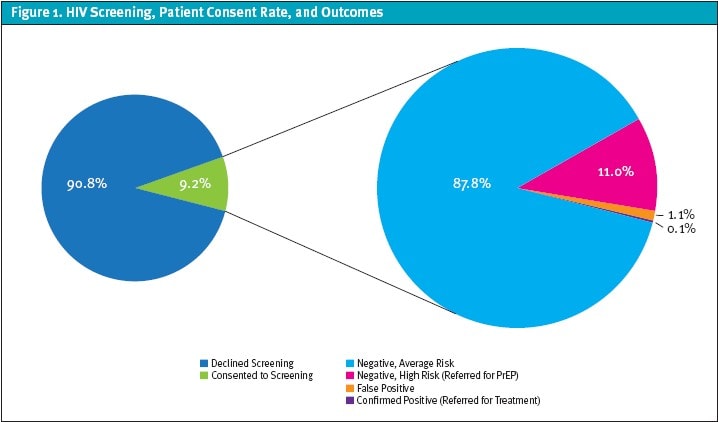
False positive results were attributed to multiple factors. Eighteen percent of false positives were attributed to sample contamination at a specific site. Our investigation into this revealed that the countertop used for processing specimens had been contaminated with positive control fluid, leading to a high false positive rate at that one location. Additional causes, as identified in the test kit’s package insert, included incorrect storage of test kits, specimens containing elevated levels of triglycerides, and specimens from patients with concurrent herpes simplex virus infection.2
The D2D times revealed no statistically significant difference between the test sites and control sites, with test sites averaging just 1.7 minutes longer (p=0.37). This suggests that there was no strain on operational efficiency. Because testing began during triage, the results were ready for the provider to discuss with the patient by the start of their evaluation, minimizing the impact of this workflow on D2D time.
Conclusion
The COVID-19 pandemic created a gap in access to HIV screening and prevention services for at-risk populations. This quality program achieved a 9.2% consent rate, which is markedly higher than the rates seen in physician offices (0.59%), emergency departments (0.95%), and community health centers (3.74%) in the northeastern United States.3 This notably higher consent rate demonstrates the practicality and effectiveness of incorporating HIV services into the urgent care setting. With a 9.2% testing consent rate, 11% PrEP referral rate, and 0.1% HIV diagnosis, urgent care centers are essential for expanding access to timely and effective HIV prevention and surveillance.
Disclosures
A grant was received from Abbott Rapid Diagnostics, providing Determine HIV-1/2 Ab/Ag rapid tests at no expense.
Manuscript submitted September 26, 2024; accepted March 27, 2025.
References
- Hoover KW, Zhu W, Gant ZC, et al. HIV Services and Outcomes During the COVID-19 Pandemic — United States, 2019–2021. MMWR Morb Mortal Wkly Rep 2022;71:1505–1510. DOI: http://dx.doi.org/10.15585/mmwr.mm7148a1.
- Determine HIV-1/2 Ag/Ab COMBO. Package insert. Abbott Rapid Diagnostics. 2021. https://www.fda.gov/media/86959/download
- Hoover KW, Huang YA, Tanner ML, et al. HIV Testing Trends at Visits to Physician Offices, Community Health Centers, and Emergency Departments — United States, 2009–2017. MMWR Morb Mortal Wkly Rep 2020;69:776–780. DOI: http://dx.doi.org/10.15585/mmwr.mm6925a2
Author Affiliations: Erin Hunt, PA, FCUCM, Northwell Health GoHealth Urgent Care. Megan Greger, PA, Northwell Health GoHealth Urgent Care. Neal Shipley MD, MBA, FACEP, Northwell Health GoHealth Urgent Care.
Read More
- New Regimens, Similar Conclusions for PrEP. Do They Change Anything for Urgent Care?
- Insidious Unilateral Axillary Swelling in a Patient with Untreated HIV: A Case Report
- New Data on HIV Infection Are Underwhelming—and Highlight the Need for Urgent Care Involvement

