Urgent message: The depth of COVID-19 testing data specific to the pediatric urgent care market provides insights into the capability of the broader urgent care industry to play a significant role in public health in the United States.
David J. Mathison, MD, MBA
It’s easy to forget how 24 months ago the urgent care industry was amidst one of the worst influenza seasons in recent memory. Then in February 2020, the first cases of COVID-19 were identified in the United States and the subsequent impact of the SARS-CoV-2 virus changed urgent care for the foreseeable future. When many primary care offices shut their doors and EDs were overwhelmed, urgent care stood as a pillar, not only to care for the sick and injured, but also functioning more broadly as community servants, public educators, and leaders in infection containment.
Now 2 years later, UC continues to be at the forefront for access, evaluation, and guidance during the ongoing pandemic. However, the trends and patterns to seek care at UC centers have differed significantly for the pediatric population than for adults. Transmission, time-to-vaccination, return-to-activity, modes of exposure, and severity of disease have all been fundamentally different for children. For both kids and adults, however, COVID testing has been the most powerful driver of UC volume during the pandemic, ebbing from gentle streams to volatile tidal waves.
The following narrative is a reflection on COVID management in pediatric urgent care (PUC), highlighting the social dynamics with this vulnerable population.
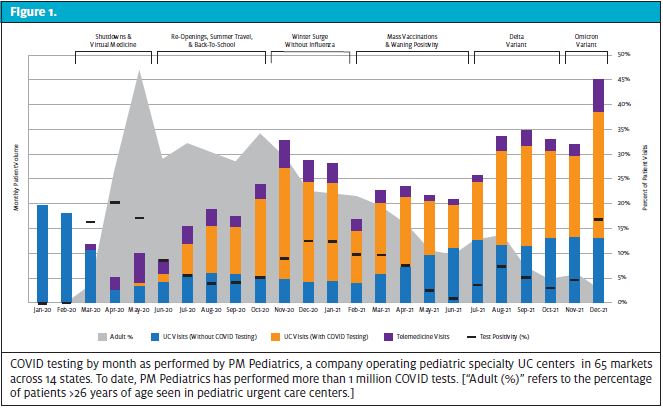
PHASE I – SHUTDOWNS, TESTING AND VIRTUAL MEDICINE (February – May 2020)
The first pediatric case of COVID-19 was identified in late February at a Seattle Children’s pediatric UC, foreshadowing the role urgent care would play as an access point throughout the pandemic. As cases began to appear in the U.S., demand for testing spiked. Yet, at this time, definitive SARS-CoV-2 testing was offered only through local health departments.
In early March, testing became more widely available through hospitals as well as national labs such as LabCorp and Quest. Soon thereafter, the World Health Organization officially declared the pandemic and the Center for Medicare & Medicaid Services paved the way for expanded use of telehealth. This also marked the introduction of the Coronavirus Aid, Relief, and Economic Security Act (CARES) as stay-at-home orders were initiated in most states. Schools shifted to virtual classrooms and child sports/activities were shutdown. By late March, the U.S. already led the world in the number of confirmed cases. Patients with mild flu-like symptoms were advised to home quarantine without testing, perhaps underestimating the true prevalence of disease. Many diagnoses of COVID-19 were made clinically via telemedicine as more UC clinicians embraced virtual care as a viable alternative. As essential workers became infected and small businesses reopened, testing demand spiked and molecular nucleic acid amplification testing (NAAT), specifically PCR, became the gold standard.
What About Children?
For the pediatric population, the initial testing demand was virtually absent. Without routine child-to-child interactions in school or sports, the transmission of COVID along with other seasonal circulating viruses (that could cause COVID-like symptoms) was extremely limited. Additionally, several studies suggested children were not as capable of transmission as their adult counterparts.
Many pediatric UCs pivoted to offer limited care for adults not dissimilar to their pediatric hospital counterparts who were offloading adult care from the general EDs. The lack of child illness, injury, and testing demand drove PUC volume down by 80% in some markets, as even true emergencies stayed at home because of apprehension of contagion. Telemedicine flourished and pediatric providers began treating conditions remotely without complete diagnostic assessment, such as otitis externa and urinary tract infections.
As kids spent more time outside in late Spring, injuries started to become more prevalent, though without the aggressive play of competitive sports injuries remained less common than in previous years. Acuity of visits increased as patients often presented with prolonged symptoms and a greater percentage of patients required procedural care.
May 2020 marked a surge in demand for antibody testing, which was appealing for patients who had recent flu-like illnesses or exposures to infected persons at a time when acute testing was unavailable. In June 2020, as some schools concluded their calendar years, children began looking towards a summer season that would be far different than years’ past.
PHASE II – REOPENINGS, SUMMER TRAVEL, AND BACK-TO-SCHOOL (July – September 2020)
As outdoor summer season approached, some stay-at-home orders were lifted, workplaces reopened and some states discarded mask mandates. Outbreaks began to occur in scattered pockets nationally after the July 4 holiday. While testing was more widely available, would-be patients remained weary to venture into indoor spaces for evaluation or testing. In response, many UC clinics opened drive-up or tent-based testing options.
As social interactions increased and restrictions eased, the COVID positivity rates climbed, spurring testing demand and straining national labs. PCR turnaround times increased significantly and conservative CDC guidelines forced patients to have extended quarantines for mild illness or peripheral exposures.
While local municipalities operated large-scale testing centers, UCs were often preferred because of added convenience, the opportunity for COVID-related counseling, and faster results. Rapid antigen tests gained popularity because of their convenience and large organizations advocated for frequent, even daily, antigen testing as a means to prevent outbreaks within. However, the ability to scale rapid antigen testing in non-healthcare settings was difficult to operationalize because of cost, test availability, staffing, and education required to administer tests effectively. While antigen tests had high sensitivities for symptomatic patients, these tests were shown to be less sensitive for screening asymptomatic patients.
What About Children?
Nationally, about two-thirds of summer camps were closed and children were forced to adapt to a different sort of summertime recess. The WHO announced that the virus could likely be spread via airborne droplets, which raised concern for children resuming normal activities. Schools evaluated filtration systems and planned for outdoor classrooms to accommodate the continued uncertainty about indoor safety. Cases began to rise in the summer as kids increasingly returned to play, though the overall prevalence was low in total number and percentage relative to adults. A significant portion of pediatric testing was guided by possible exposures to positive adults or for recreational travel, rather than for acute illness.
A new inflammatory disease pattern was recognized, later termed multisystem inflammatory syndrome in children (MIS-C), and was noted to occur most commonly following COVID in children, sometimes even after an initially asymptomatic infections.
In the Fall, many school systems opened virtual-only or with hybrid models. While some were able to develop surveillance testing strategies, this was not commonplace (especially for large public school systems). PCR was generally the most accepted test for return-to-school clearance after exposures, travel, and other high-risk activities.
PHASE III – WINTER VIRUS SURGE WITHOUT INFLUENZA (October 2020 – January 2021)
As the workforce returned from home and more states advanced their reopening plans, winter weather set in and indoor gatherings increased, leading to rises in COVID positivity rates nationally. In October 2020, then-President Trump was hospitalized with COVID-19 and the FDA approved monoclonal antibody (mAB) therapy as an option for high-risk infections.
Despite low rates of circulating non-COVID viral disease and almost no influenza, the demand for COVID testing surged. Many states reversed reopening plans because of the rapidly rising numbers of cases. With increased testing demand, turnaround times for PCR tests slowed again, often frustrating families as they tried to navigate holiday travel plans.
In December 2020, vaccine options first became available for essential workers, offering hope for reprieve in the new year.
What About Children?
Most schools continued hybrid or virtual models. Mask mandates for children who did return to in-person learning were generally stringent and sports were often restricted, which perpetuated limited transmission in children. Cases that did occur in children were most commonly attributable to adult family members or teachers, rather than child-to-child transmissions. The demand for antigen testing increased to assess household risk and guide quarantine planning, especially for parents of young children where separate household quarantines were not an option. Some schools operating in-person or hybrid models developed surveillance testing to screen for prevalence of disease in their specific communities; however, this was difficult to operationalize for many large public school systems that remained virtual throughout the winter.
PHASE IV – MASS VACCINATIONS & WANING POSITIVITY (February – June 2021)
As multiple vaccination options became available to the general population, COVID rates fell, especially in urban areas. National labs improved COVID PCR turnaround times, allowing for easier decision-making around clearance for work, school, and travel. June 2021 featured the lowest COVID positivity rates (<1% in many areas), prompting optimism for summer.
What About Children?
With declining rates, many school districts reopened in Spring for in-person education. Warmer weather saw many sports leagues resume, often omitting mask requirements for outdoor activities. Rates of other circulating viruses continued to be low, although were more prevalent in the daycare groups where children <3 years old were inconsistently able to wear masks. While COVID was on the decline, RSV was on the rise with spikes in activity 5 to 6 months after the typical seasonal pattern. Some schools initiated pooled testing; this allowed for identification of classrooms at risk—an effective strategy when prevalence is low.
In May 2021, vaccines became available for the 12- to 15-year-old population. Injuries and “typical” UC complaints became more frequent, especially for middle and high school students. Camps that were previously closed planned for full seasons with the addition of strict testing protocols. Summer school resumed and school districts planned for full reopenings in the Fall.
PHASE V – DELTA VARIANT (July – November 2021)
Typical summertime enteroviruses and other respiratory viruses began to circulate with increased social interactions. In July 2021, the Delta variant emerged and began surging around the country. While adults continued to be the most common vectors, child-to-child transmission also increased, particularly among older children and adolescents. Travel and return-to-camp requirements prompted testing spikes. Antigen testing became more generally accepted for workplace clearance in some industries.
In September 2021, President Biden encouraged organizations to require vaccinations. Many hospitals, universities, and large employers listened and began introducing such mandates as did other service-based industries and select school systems. As the weather cooled, rhinovirus and influenza rates increased, particularly in the close quarters of college campuses. In late fall, the Food & Drug Administration recommended booster vaccines for adults.
What About Children?
In November 2021, the FDA authorized COVID vaccination for the 5- to 12-year-old population. Most schools reopened for in-person education, often without formal surveillance testing or virtual options. The Massachusetts model to stay-in-school after a masked exposure became popular and expanded to other jurisdictions. Students could stay in the classroom if the school performed frequent postexposure antigen testing on-site.
While positivity rates generally declined after the August to September spikes, cold weather season and increased participation in sports led to a moderate rise in pediatric COVID rates. A fragile unvaccinated child population remained vulnerable as the country moved to complete reopening without prevention measures necessary to protect children. Children began getting vaccinated in November but were not able to complete a two-vaccine series prior to the surge from the Delta variant. While child-to-child spread was still limited, disease spread became more prevalent in adolescents, especially among those who were unvaccinated.
OMICRON VARIANT (December 2021 – January 2022)
In December 2021, the Omicron variant emerged from South Africa and rapidly spread to the U.S. Relative to prior variants, Omicron spread extremely quickly, leading to greater positivity rates than ever. With large scale testing centers mostly retired, the impact on urgent care was overwhelming; this impact was exacerbated by demands for holiday testing. Fears that Omicron was more transmissible were confirmed by several studies, including a Japanese report showing Omicron was 4.2 times more contagious than Delta and more likely to evade the immunity from both infection and vaccination.
Despite its infectivity, Omicron thankfully seemed to cause less severe disease in most, despite unprecedented numbers of hospitalizations in the U.S. By late December 2021, Omicron accounted for up to 90% of U.S. COVID infections. The FDA continued to further other protection measures, approving the use of an oral medications (eg, molnupiravir) for use in mild-to-moderate COVID disease in adults, and then approving booster vaccinations for adolescents.
What About Children?
With infants and toddlers fully unvaccinated and the 5- to 12-year-old population only beginning their series, the school-aged patients were especially susceptible to this surge. Unlike previous SARS-CoV-2 strains, Omicron was noted to cause more disease in the nasopharynx and upper airway, leading to more cases of “COVID croup” in toddlers. In daycares, due to decreased mask use, increased infectivity, and lack of vaccine approval, Omicron spread more readily than previous strains where child-to-child transmission was rare. Despite increases in pediatric hospitalizations, the severity of disease in children continued to be mild and was similar to other winter-time viral respiratory pathogens.
WHAT’S NEXT?
The role of COVID testing for PUC centers has been essential. Urgent care-driven testing allows for the benefits of clinician interaction such as counseling on recovery and isolation, evaluation for complications, and treatment of comorbidities. While daycares, schools, and recreational leagues have often created their own (sometimes divergent) policies, UC has been a stalwart in directing consistent, evidence-based management for testing and clearance.
Upcoming years may see a shift from identifying COVID-19 to distinguishing the respiratory pathogens that are truly more dangerous from those associated with lower severity, such as the other coronaviruses. Urgent care’s role will be increasingly to help in the diagnostic assessment of respiratory conditions and to mitigate disease spread, whether it be RSV, COVID variants, or the flu, in each community. While home testing has soared in availability, it is not clear how much this will supplant seeking medical attention in the years to come.
For pediatrics, the downstream effects of the virus are certain to continue. As research focuses on future vaccines and antivirals, the impact on children will likely lag behind the adult population. While the severity of disease in children has been undoubtedly mild, kids could be a vector for transmission and mutation and will therefore be a critical group to attend to if we hope to truly end this pandemic.
For now, new variants are circulating, flu season is underway, and child COVID vaccinations are in their infancy. The coming months will stir ongoing controversy about vaccine mandates, antiviral options, appropriate use of diagnostic testing, lengths of isolation periods, and how to live with endemic COVID-19. For pediatrics, the trends will always be somewhat different from adults because of behavior, regulation, and the effects of the virus itself. It will remain important for urgent care to consider the unique characteristics of the pediatric population in order to make the best decisions both for our individual patients and also the health of the entire population.
David J. Mathison, MD, MBA is Vice President, Clinical Operations, South Atlantic for PM Pediatrics and a pediatric emergency physician.

