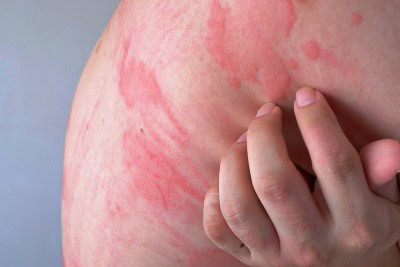
The Food and Drug Administration (FDA) has approved oral remibrutinib, a new drug product, as a second-line treatment for chronic spontaneous urticaria (CSU). Remibrutinib has a unique mechanism to block the activity of Bruton’s tyrosine kinase (BTK), stopping a key pathway of the immune response in CSU, according to a manufacturer press release. The condition is thought to be caused by immune dysregulation, and it is believed that once activated, BTK leads to the release …
Read More