Published on
Urgent Message: Lymphadenopathy in patients living with HIV is most commonly related to HIV infection, but it may also be related to many forms of systemic infection or malignancy. Definitive diagnosis is most often achieved by fine-needle aspiration.
Hana Kusumoto, MD, MPH; Lindsey E. Fish, MD
Citation: Kusumoto H, Fish LE. Insidious Unilateral Axillary Swelling in a Patient with Untreated HIV: A Case Report. J Urgent Care Med. 2025; 19(8):31-35
Key words: Diffuse Large B Cell Lymphoma, Human Immunodeficiency Virus, Lymphadenopathy
Abstract
Introduction:Lymphadenopathy (LAD) can result from a variety of conditions. While in some cases LAD may be reactive and self-resolving, it may also represent time-sensitive diagnoses including cancer and human immunodeficiency virus (HIV).
Clinical Presentation: A 41-year-old man presented to urgent care (UC) with complaints of new swelling in his right axilla for the previous 2 weeks and was concerned for an abscess. His past medical history included HIV, for which he was not taking antiretroviral treatment (ART). He denied pain in the axilla, fevers, night sweats, and weight loss.
Physical Exam: On exam, a 4x5cm, well-circumscribed mass in the right axilla was noted without associated erythema, fluctuance, or tenderness.
Testing: A point-of-care ultrasound (POCUS) exam showed no fluid collection at the site of axillary swelling.
Case Resolution: Computed tomography (CT) imaging performed 3 days later showed an ill-defined axillary mass as well as scattered, enlarged thoracic and abdominal lymph nodes with mild splenomegaly. His absolute CD4 count and HIV viral load returned at 76 cells/µL(reference range: 496-1,647 cells/µL) and >216,000 copies/mL (reference range: ≤0 copies/mL), respectively. When the axillary lesion was biopsied, pathology returned showing diffuse B-cell lymphoma. The patient was started on chemotherapy, and ART was resumed.
Conclusion: HIV-related diseases, particularly in patients not taking ART, include a wide array of secondary infectious and non-infectious conditions. New LAD in HIV-positive patients requires a broad differential diagnosis and biopsy to confirm the cause.
Introduction
Lymphadenopathy (LAD) can indicate a wide variety of pathology ranging from reactive and self-limited hyperplasia, which is most common in the acute setting, to serious, systemic illness.1 For patients living with human immunodeficiency virus (HIV) specifically, LAD can be a manifestation of HIV infection itself or may be indicative of insidious conditions such as cancer or other infections. Differentiating the etiology of LAD is clinically challenging and generally requires an extensive diagnostic evaluation.2 Given that an estimated 1.2 million people in the United States live with HIV, many of whom are not yet diagnosed,3 clinicians in urgent care (UC) centers should use extra caution when approaching the assessment of possible LAD in patients living with HIV as it is more likely a sign of serious disease.
Clinical Presentation
A 41-year-old man presented to UC with complaints of swelling in his right armpit, which he had noticed 2 weeks prior. In the 2 days before his visit, he reported that the swelling had increased in size, and he had concerns for an abscess. He denied redness or pain at the site of swelling and denied fevers, chills, or other infectious symptoms. He denied any swelling elsewhere, including in the groin or neck. He denied smoking and drug use. His review of systems was negative for weight loss, night sweats, cough, dyspnea, and hemoptysis. His past medical history was most notably positive for HIV, which was diagnosed 8 years prior. Due to limited health literacy and a lack of health insurance, he had stopped antiretroviral therapy (ART) 6 years before the visit.
Physical Exam Findings
The patient’s vital signs were normal, and he was afebrile: temperature was 36.4˚C; blood pressure was 139/82; heart rate was 95 beats per minute; respiratory rate was 16; blood oxygen saturation (SpO2) was 98%. He was overall well appearing and in no distress. In the right axilla, a 4x5cm well-circumscribed, firm mass without overlying erythema, fluctuance, or tenderness to palpation was noted. Palpation of the inguinal, cervical, and contralateral axillary regions revealed no other areas of swelling or masses. The remainder of his exam including skin, abdomen, and cardiopulmonary was also normal.
Medical Decision Making
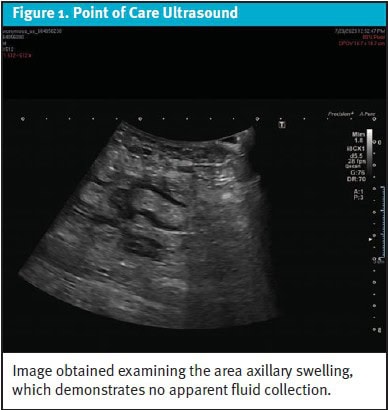
A point-of-care ultrasound (POCUS) exam of the area of swelling in the right axilla demonstrated no discrete fluid collection (Figure 1), further decreasing the probability that an abscess was responsible for the swelling. A complete blood count (CBC) was obtained, which was normal except for mild leukopenia with a total white blood cell count of 3.6 k/µL (reference range: 4.5-10.0 k/µL). Given his lack of follow-up, a CD4 t-cell count and HIV viral load were also collected, although results were not immediately available. The differential of infectious and neoplastic conditions for a patient with untreated HIV was considered including bacterial, fungal, viral, and tuberculous infections as well as various forms of lymphoma. The patient was referred for a stat thoracoabdominal computed tomography (CT) imaging and follow-up with the infectious disease clinic.
Case Continuation and Management
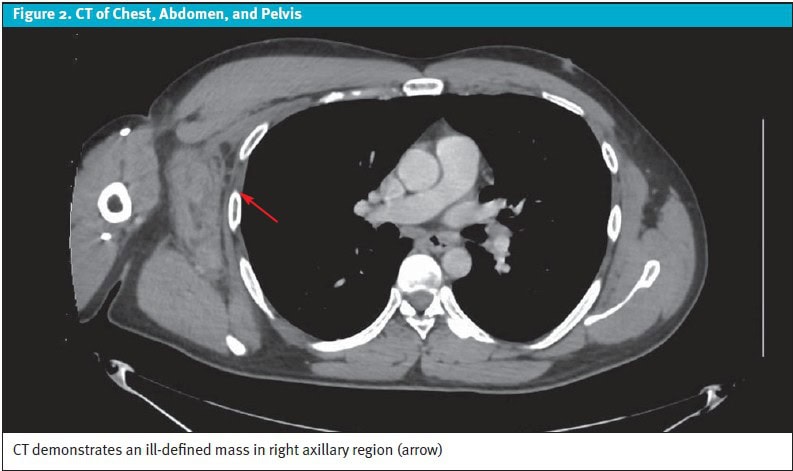
The next day, his absolute CD4 count returned at 76 cells/µL(reference: 496-1,647 cells/µL) and his HIV viral load was >216,000 copies/mL (reference: ≤0 copies/mL), confirming untreated HIV infection. The patient was seen by an infectious disease specialist to manage resuming his ART. CT imaging of the chest, abdomen, and pelvis with intravenous contrast was performed 3 days later, which confirmed that there was no drainable axillary fluid collection. The CT, however, did demonstrate an ill-defined enhancing mass in the right axilla (8.4×3.8×7.8 cm) (Figure 2) as well as scattered, pathologically enlarged thoracic and abdominal lymph nodes, and mild splenomegaly.
Final Diagnosis
Ultimately, a biopsy of the axillary mass was obtained, and the final pathology revealed diffuse large B-cell lymphoma (DLBCL). The patient underwent inpatient positron emission tomography imaging and induction chemotherapy. The patient had a good response to chemotherapy, and after 6 cycles, repeat imaging performed approximately 1 year after diagnosis revealed no evidence of disease—indicating complete remission.
Discussion
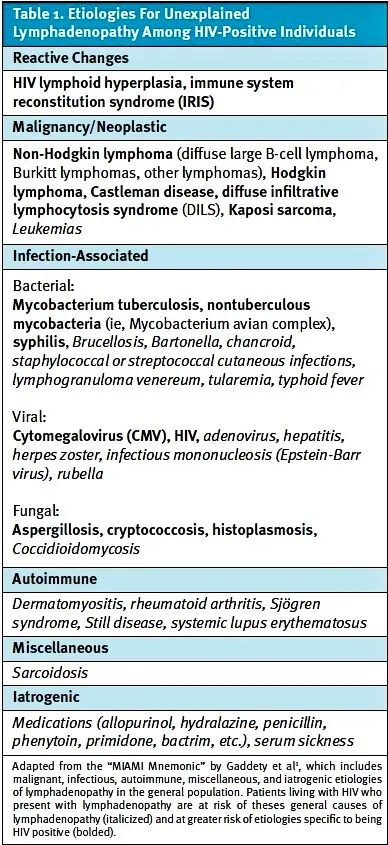
LAD can represent myriad conditions including benign and self-limited processes (ie, reactive LAD), malignancy, autoimmune disorders, and infectious etiologies.1 For patients with HIV, however, LAD is more likely to represent other etiologies compared to the general population (Table 1) including specific malignancies4 and mycobacterial infections.5 For this reason, HIV testing should be considered as part of the initial diagnostic approach for unexplained LAD in patients.1
LAD, when present in patients living with HIV, is commonly a manifestation of HIV infection itself with 40-50% of lymph node biopsies in such patients demonstrating reactive lymphoid hyperplasia related to HIV.6,7 This occurs due to the virus’ lymphotropic properties and most often occurs in the setting of acute HIV infection.8
Secondary infectious causes of LAD in HIV positive patients are most often due to mycobacterial infections.7 In endemic countries, Mycobacterium tuberculosis is the most common mycobacterial infection, whereas nontuberculous mycobacteria, (ie, mycobacterium avian complex (MAC)) is more often seen in industrialized countries.2Fine needle biopsy showing caseating necrosis and ill-formed granulomas are the hallmark findings of Mycobacterium tuberculosis.7 Other infectious etiologies that can produce pathologic LAD among HIV infected individuals include cryptococcus, histoplasma, aspergillis, cytomegalovirus, and syphilis.2,6,9 Lastly, opportunistic infections can also precipitate LAD when ART-naïve patients initiate therapy—a phenomenon termed immune-system reconstitution syndrome.10
Neoplastic causesof LAD in patients living with HIV are primarily related to various forms of lymphoma and constitute the final diagnosis in 42% of cases of HIV-related LAD, although this rate is less in regions with higher burdens of opportunistic infections.6 Non-Hodgkin lymphoma (NHL) is the most common HIV associated malignancy, with DLBCL constituting the most common subtype found on biopsy.11 In the past 3 decades, however, the incidence of Burkitt and Hodgkin lymphomas among HIV-positive patients with LAD has increased, and survival of patients diagnosed with HIV-associated lymphoma has improved.12,13
Castleman disease represents a spectrum of pathologic LAD that shares histological features; it is variably classified as unicentric or multicentric depending on the number of lymph nodes affected.14 Castleman disease, while rare, has higher prevalence among patients with HIV and human herpesvirus 8 infection.15 Castleman disease itself does not meaningfully impact life expectancy, but afflicted patients are at an increased risk for lymphoma, autoimmune disorders, chronic lung disease, or even compression of nearby structures from enlarged lymph nodes, which may impact survival without appropriate surveillance.16
Given the vast array of etiologies of LAD in patients living with HIV, clinical suspicion for more serious causes of LAD, especially if multifocal, persistent, and progressive, is warranted. Laboratory testing, including absolute CD4 count, HIV viral load, and CBC, as well as imaging and biopsy, usually with fine-needle aspiration (FNA) 2,17,18 is typically required to confirm the cause of LAD. While coordinating this from UC may not be achievable, it is important that patients be informed of the high likelihood of a serious cause for the LAD and efforts are made to coordinate expedient continuation of the work-up.
Conclusion
In UC settings, patients may present with swelling that is consistent with LAD. It is crucial to inquire about a patient’s HIV status in cases of LAD that are not clearly reactive and benign. Patients presenting with concerning LAD and a prior diagnosis of HIV, regardless of their use of ART, require a thorough history and exam. However, clinically confirming the cause of LAD is generally not feasible, and a FNA biopsy is usually required to ascertain which of the many possible etiologies for LAD is causal.
Ethics Statement
Verbal consent for publication of this case was obtained from the patient.
Takeaway Points
- Lymphadenopathy in patients living with HIV is most commonly related to HIV infection but may also be related to many forms of systemic infection or malignancy.
- Even in the absence of diffuse Lymphadenopathy or systemic symptoms, Lymphadenopathy in patients with HIV commonly represents serious diseases, including lymphoma and mycobacterial infection.
- Many patients with HIV are undiagnosed, and HIV testing should be pursued in patients with apparent pathologic LAD.
- Determining the definitive diagnosis for the cause of suspected HIV-related LAD requires a biopsy, which is most often achieved by FNA.
Manuscript submitted December 20, 2024; accepted March 27, 2025.
References
- Gaddey HL, Riegel AM. Unexplained Lymphadenopathy: Evaluation and Differential Diagnosis. Am Fam Physician. 2016;94(11):896-903.
- Glushko T, He L, McNamee W, Babu AS, Simpson SA. HIV Lymphadenopathy: Differential Diagnosis and Important Imaging Features. American Journal of Roentgenology. 2021;216(2):526-533. doi:10.2214/AJR.19.22334
- Centers for Disease Control and Prevention. Estimated HIV Incidence and Prevalence in the United States, 2018–2022.; 2024. Accessed December 9, 2024. https://www.cdc.gov/hiv-data/nhss/estimated-hiv-incidence-and-prevalence.html
- Kimani SM, Painschab MS, Horner MJ, et al. Epidemiology of haematological malignancies in people living with HIV. Lancet HIV. 2020;7(9):e641-e651. doi:10.1016/S2352-3018(20)30118-1
- Sun L, Zhang L, Yang K, et al. Analysis of the causes of cervical lymphadenopathy using fine-needle aspiration cytology combining cell block in Chinese patients with and without HIV infection. BMC Infect Dis. 2020;20(1):224. doi:10.1186/s12879-020-4951-x
- Bogoch II, Andrews JR, Nagami EH, Rivera AM, Gandhi RT, Stone D. Clinical predictors for the aetiology of peripheral lymphadenopathy in HIV-infected adults. HIV Med. 2013;14(3):182-186. doi:10.1111/j.1468-1293.2012.01035.x
- Nag D, Dey S, Nandi A, Bandyopadhyay R, Roychowdhury D, Roy R. Etiological study of lymphadenopathy in HIV-infected patients in a tertiary care hospital. J Cytol. 2016;33(2):66. doi:10.4103/0970-9371.182518
- Lederman MM, Margolis L. The lymph node in HIV pathogenesis. Semin Immunol. 2008;20(3):187-195. doi:10.1016/j.smim.2008.06.001
- Kamana NK, Wanchu A, Sachdeva RK, Kalra N, Rajawanshi A. Tuberculosis is the leading cause of lymphadenopathy in HIV-infected persons in India: results of a fine-needle aspiration analysis. Scand J Infect Dis. 2010;42(11-12):827-830. doi:10.3109/00365548.2010.498016
- Berman EJ, Iyer RS, Addrizzo-Harris D, Ko JP. Immune-reconstitution syndrome related to atypical mycobacterial infection in AIDS. J Thorac Imaging. 2008;23(3):182-187. doi:10.1097/RTI.0b013e3181653c25
- Gessese T, Asrie F, Mulatie Z. Human Immunodeficiency Virus Related Non-Hodgkin’s Lymphoma. Blood Lymphat Cancer. 2023;13:13-24. doi:10.2147/BLCTT.S407086
- Carbone A, Vaccher E, Gloghini A. Hematologic cancers in individuals infected by HIV. Blood. 2022;139(7):995-1012. doi:10.1182/blood.2020005469
- Ramaswami R, Chia G, Dalla Pria A, et al. Evolution of HIV-Associated Lymphoma Over 3 Decades. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2016;72(2):177-183. doi:10.1097/QAI.0000000000000946
- Fajgenbaum DC, Shilling D. Castleman Disease Pathogenesis. Hematology/Oncology Clinics of North America. 2018;32(1):11-21. doi:10.1016/j.hoc.2017.09.002
- Simpson D. Epidemiology of Castleman Disease. Hematology/Oncology Clinics of North America. 2018;32(1):1-10. doi:10.1016/j.hoc.2017.09.001
- Van Rhee F, Oksenhendler E, Srkalovic G, et al. International evidence-based consensus diagnostic and treatment guidelines for unicentric Castleman disease. Blood Advances. 2020;4(23):6039-6050. doi:10.1182/bloodadvances.2020003334
- Reddy DL, Venter WDF, Pather S. Patterns of Lymph Node Pathology; Fine Needle Aspiration Biopsy as an Evaluation Tool for Lymphadenopathy: A Retrospective Descriptive Study Conducted at the Largest Hospital in Africa. PLoS One. 2015;10(6):e0130148. doi:10.1371/journal.pone.0130148
- Muyanja D, Kalyesubula R, Namukwaya E, Othieno E, Mayanja-Kizza H. Diagnostic accuracy of fine needle aspiration cytology in providing a diagnosis of cervical lymphadenopathy among HIV-infected patients. Afr Health Sci. 2015;15(1):107-116. doi:10.4314/ahs.v15i1.15
Author Affiliations: Hana Kusumoto, MD, MPH, University of Colorado School of Medicine. Lindsey E. Fish, MD, Denver Health and Hospital Authority and University of Colorado School of Medicine. Authors have no relevant financial relationships with any ineligible companies.
Download the article PDF: Insidious Unilateral Axillary Swelling in a Patient with Untreated HIV: A Case Report

