Published on
Urgent message: Urgent care centers may offer opportunities to provide preexposure prophylaxis (PrEP) services for HIV due to large numbers of patients seeking testing and treatment for sexually transmitted infection. In the same sense, the urgent care center may also serve as an important ally in providing referrals for patients not currently linked to primary care services.
Yeow Chye Ng, PhD, FNP-BC, NP-C, CPC, AAHIVE, Jack J. Mayeux, MSN, APRN, NP-C, and Thuy Lynch, PhD, RN.
Introduction
Preexposure prophylaxis (PrEP) services for HIV are not currently offered in a majority of urgent care settings in the United States. Common reasons include the complexity of the treatment protocols and concern about providers being reimbursed for the time spent providing these services. Here, we describe a process for initiating PrEP services and offer a list of service codes providers can use when offering services.
The concept of PrEP was first approved by the U.S. Food and Drug Administration as a medication option to reduce HIV infection.1,2 Since that time, many primary care clinicians have begun offering PrEP services.3 PrEP is a once-a-day medication regimen recommended for people not currently infected by HIV and who participate in risky sexual behavior. PrEP can reduce the risk of contracting the HIV infection up to 99% if the patient adheres to a strict medication regimen.4-7 Behavioral and lifestyle changes determine the duration of continuum for PrEP care.
Not everyone is considered a likely candidate due to lab testing requirements prior to the initiation of PrEP. Lab tests include screening all prospective patients for sexual transmitted infections (STI); HIV; and hepatitis B and C; and checking for renal insufficiency. It is advisable to ensure that testing for STIs be conducted in a uniform fashion—ie, testing for the same STIs in the same fashion for each patient as indicated. If any of these tests are recorded as positive, the patient will not be able to begin PrEP.8 From 2012 through 2015, it was estimated that 79,000 people in the U.S. received PrEP services.9
PrEP treatment is a vital part of HIV prevention. This prevention treatment process mirrors other chronic disease management plans in which patients are required to seek follow-up care services, lab evaluations, and medication refills. Guidelines for clinicians who initiate PrEP are widely available from several government agency websites.8,10,11 Unfortunately, a large percentage of medical providers (approximately 34%) are not aware of the PrEP treatment process.12
Due to busy schedules and time constraints, patients are constantly seeking more convenient hours to initiate their medical or follow-up care. For urgent care facilities that operate 7 days a week and have access to in-house lab testing, same-day PrEP services are certainly a feasible option. For facilities that do not offer in-house lab testing, PrEP services can also be considered with scheduled follow-up visits upon verification of lab results.
Studies have revealed a 2.5-fold increase in overall urgent care utilization between 2010 and 2014.13 Within the same clinical setting, researchers reported an increase in requested services for STI testing. STI is considered one of the major risk factors for patients contracting HIV. Treatment and services were sought by patients with an average age of 30 years. This age group also is the third-highest age group to be newly diagnosed with HIV, as presented by a surveillance report from the Centers for Disease Control and Prevention.14
What You Need to Know Before Offering and Initiating PrEP Services
Know your local community HIV/STD resources
Providers in the urgent care center should establish a collaborative partnership with the local community HIV prevention and treatment facility. Many of these facilities are pioneers in managing HIV and preventive care. They also have resources that could assist the urgent care center in expanding PrEP services. By offering PrEP services, the urgent care setting can meet important community and patient needs. The urgent care facility can accept and accommodate these walk-in patients during off hours.
PrEP treatment protocol
Embracing the current PrEP treatment protocol will provide a seamless transition for patients regardless of which treatment facility they choose to initiate their follow-up care. A majority of HIV prevention centers have adopted the same treatment guidelines from the CDC. Table 1 provides a summary of the recommended laboratory testing prior to initiating PrEP services.
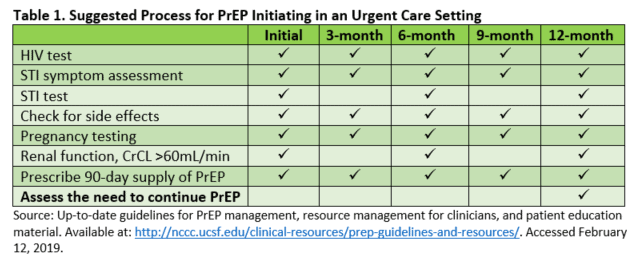
Screening for STIs, initiating PrEP, follow-up testing
Current CDC treatment guidelines and PrEP protocol begin with screening for acute HIV infection and HIV laboratory testing. Additional testing includes screening for STIs and hepatitis B and C, pregnancy testing, and assessment of renal function being at eCrCl >60 mL/min.8
Once PrEP has been initiated, laboratory testing and screening for medication side effects should take place at 1 month with subsequent follow-up and testing being accomplished every 3 months. Testing for STI and assessment of renal function can be delayed for every 6 months, unless otherwise needed. PrEP refills should be limited to no more than 90 days.8
PrEP medications
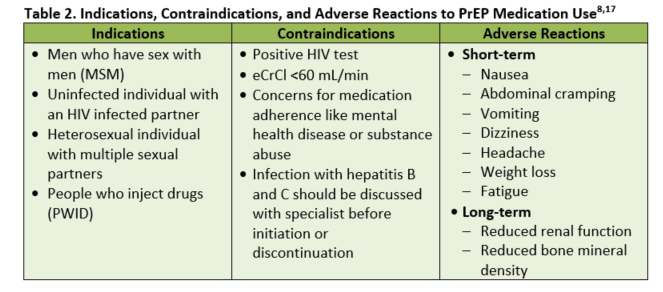
Currently, within the U.S., the only medication proven safe and effective is a fixed-dosed combination medication containing tenofovir disoproxil fumarate (TDF) 300 mg and emtricitabine (FTC) 200 mg.8 This medication is a once-daily dose recommended for men who have sex with men (MSM), heterosexually active men and women, and people who inject drugs (PWID). While this combination medication is preferred, TDF alone has been proven effective and can be used in PWID and heterosexually active men and women. Only once-daily dosing should be used for PrEP, and no other antiretroviral medications should be used in place of or in addition to TDF/FTC. PrEP should not be provided to any individual who has not undergone necessary testing and is not under the provider’s care.
PrEP medication drug interactions
While the effects of PrEP medications, TDF/FTC, have not been studied in combination against other drugs, they have been evaluated individually.15,16 In studies of TDF, no significant effect or dose adjustment was found necessary when taken with buprenorphine, methadone, and oral contraceptives.
The serum concentrations of TDF and/or the following drugs may be increased, requiring monitoring for dose-related renal toxicities: antivirals, aminoglycosides, high-dose NSAIDs, or other drugs that reduce renal function. Additionally, ledipasvir/sofosbuvir may increase TDF concentrations and will require monitoring for toxicity. FTC currently has no data on any of the medications listed, with the exception of ledipasvir/sofosbuvir, which has no significant side effect.
Side effects of PrEP
- Short-term: Some of the short-term side effects include nausea, abdominal cramping, vomiting, dizziness, headache, weight loss, and fatigue.17 Many of these side effects present within the first 2 weeks of taking the medication and often resolve within a few weeks.
- Long-term: The two most common concerns with long-term use include changes in renal function and bone mineral density (BMD); however, the actual effect of PrEP on renal function and BMD are unclear and difficult to assess.8,17 Many candidates for PrEP carry risk factors (eg, substance abuse and lack of exercise) or conditions (eg, diabetes) which can affect their bodily systems aside from PrEP use. Additionally, while antiretroviral medication containing TDF/FTC has been observed to decrease BMD in HIV-infected individuals, it is unclear if this decline would have been observed in HIV-negative individuals and those taking fewer antiretrovirals.8
While many studies have measured the effect of TDF/FTC on renal function, the results are varied concerning the medications’ impact. There is a risk of kidney damage, but PrEP trials have shown low rates of creatinine elevations.17 Due to the risk of kidney damage, the need to monitor renal function throughout the course of PrEP treatment remains vital.
PrEP is not for everyone
Not all patients are candidates for PrEP. This is especially true when medication adherence is a major concern. Occasionally, a provider may also encounter patients who may benefit from mental health services or substance abuse services. In this situation, the provider will need to document in the medical records valid reasons for not offering the patient PrEP. This should be followed by a documented referral made by the medical provider for the patient.
Coding and billing
From a business and professional perspective, the mechanism of reimbursement plays a significant role in how a business entity may choose to offer any medical services. PrEP services are no different. Providers will need to collaborate with their billing department to understand the complexity of lab testing requirements for initiating PrEP. It is also vital to establish a standard template that considers patient work flow and includes the point-of-care lab testing services that are available in the clinic. Detailed documentation is critical to match with services performed.
Commonly used service codes that may be helpful for urgent care providers include:
- Evaluation and management (E/M) coding: New patients: 99203-99205; Established patients: 99213-99214
- Preventive medicine Current Procedural Terminology (CPT) codes: Some patients may benefit from having one-on-one preventive medicine counseling sessions prior to receiving a prescription for PrEP. In such a scenario, the provider may use CPT codes 99401-99404. This is based on the amount of time providers spend with the patient
- Suggested PrEP counseling ICD-10 codes: Z20.2 Contact with and (suspected) exposure to infections with a predominantly sexual mode of transmission; 4 Encounter for screening for human immunodeficiency virus [HIV]; Z11.3 Encounter for screening for infections with a predominantly sexual mode of transmission
- Prolonged non─face-to-face care: In some situations, medical providers may use CPT 99358-99359. This usually occurs when providers receive a reactive test of lab results that require additional time to review and coordinate care for the patient. Such coordination does not require the patient to be present
NASTAD, formerly known as the National Alliance of State and Territorial AIDS Directors, which represents public health officials who administer HIV and hepatitis programs in the U.S. and around the world, has a billing and coding guide for HIV prevention on its website. Providers are encouraged to visit and learn the suggested coding guide (https://www.nastad.org/resource/billing-coding-guide-hiv-prevention).
Patient education
PrEP is a powerful HIV prevention tool. However, PrEP does not protect against other sexually transmitted infections; therefore, patient education should be delivered as part of the clinical visit protocol.
Conclusion
Entrusted with an important role in HIV prevention—and due to the large numbers of patients seeking STI testing and treatment in urgent care—providers may find both clinically important and profitable opportunities in providing PrEP services. By the same token, urgent care may also serve as an important ally in providing referrals for patients not currently linked to primary care services. Fostering PrEP services in the urgent care environment can provide an alternative solution to prevent any missed opportunities for safe and effective HIV prevention.
Citation: Ng YC, Mayeux JJ, Lynch T. Initiating PrEP services in urgent care. J Urgent Care Med. March 2019. Available at: https://www.jucm.com/initiating-prep-services-in-urgent-care/.
References
- U.S. Food and Drug Administration. FDA approves first drug for reducing the risk of sexually acquired HIV infection. Silver Spring, Maryland: US Food and Drug Administration; 2012.
- Holmes D. FDA paves the way for pre-exposure HIV prophylaxis. Lancet. 2012;380(9839):325.
- Smith DK, Mendoza MC, Stryker JE, Rose CE. PrEP Awareness and Attitudes in a National Survey of Primary Care Clinicians in the United States, 2009-2015. PLoS One. 2016;11(6):e0156592.
- Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083-2090.
- Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. New Engl J Med. 2012;367(5):399-410.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. New Engl J Med. 2012;367(5):423-434.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. New Engl J Med. 2010;363(27):2587-2599.
- Centers for Disease Control and Prevention. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States 2017 Update: Clinical Practice Guideline. 2017; https://www.cdc.gov/hiv/pdf/guidelines/cdc-hiv-prep-guidelines-2017.pdf.
- Mera R, McCallister S, Palmer B, et al. FTC/TDF (Truvada) for HIV pre-exposure prophylaxis (PrEP) utilization in the United States: 2013-2015. 21st International AIDS Conference; 2016; Durban, South Africa.
- World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. In: Organization WH, ed. Gevena2015.
- Centers for Disease Control and Prevention. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2014 Clinical Practice Guideline. 2014.
- Smith DK, Mendoza MCB, Stryker JE, Rose CE. PrEP awareness and attitudes in a national survey of primary care clinicians in the United States, 2009–2015. PLOS ONE. 2016;11(6):e0156592.
- Pearson WS, Tao G, Kroeger K, Peterman TA. Increase in urgent care center visits for sexually transmitted infections, United States, 2010–2014. Emerg Infect Dis. 2017;23(2):367-369.
- Centers for Disease Control and Prevention. HIV Surveillance Report, 2015. Vol 272016.
- Gilead Sciences. Highlights of Prescribing Information: Truvada. 2018.
- Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773-780.
- Mascolini M. Weighing risks of TDF/FTC side effects in people without HIV. Winter 2012. Available at: http://www.thebodypro.com/content/72567/weighing-risks-of-tdfftc-prep-side-effects-in-peop.html. Accessed February 12, 2019.
Yeow Chye Ng, PhD, FNP-BC, NP-C, CPC, AAHIVE is an Assistant Professor at the University of Alabama in Huntsville. Jack J. Mayeux, MSN, APRN, NP-C is a DNP student from the University of Alabama in Huntsville. Thuy Lynch, PhD, RN, is an Assistant Professor at the University of Alabama in Huntsville. The authors have no relevant financial relationships with any commercial interests.



